Mitochondrial intermembrane junctional complexes and their role in cell death - PubMed (original) (raw)
Review
Mitochondrial intermembrane junctional complexes and their role in cell death
M Crompton. J Physiol. 2000.
Abstract
A mitochondrial complex comprising the voltage-dependent anion channel (outer membrane), the adenine nucleotide translocase (inner membrane) and cyclophilin-D (matrix) assembles at contact sites between the inner and outer membranes. Under pathological conditions associated with ischaemia and reperfusion the junctional complex 'deforms' into the permeability transition (PT) pore, which can open transiently, allowing free permeation of low Mr solutes across the inner membrane. This may be a critical step in the pathogenesis of lethal cell injury in ischaemia and reperfusion. Moreover, it is argued, the degree of pore opening may be an important determinant of the relative extent of apoptosis and necrosis under these conditions. In addition, mitochondria are the major sites of action of Bax and other apoptotic regulatory proteins of the Bcl-2 family. These proteins control a mitochondrial amplificatory loop in the apoptotic signalling pathway in which cytochrome c and other apoptogenic proteins of the mitochondrial intermembrane space are released into the cytosol. There are indications that the junctional complex, or components of it, may also mediate the action of Bax, but in a way that does not involve PT pore formation.
Figures
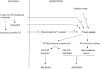
Figure 1. VDAC-ANT complexes
VDAC and ANT bind to each other and to a number of other proteins. These may, or may not, include Bax (see text). Under pathological conditions associated with ischaemia, the VDAC-ANT-CyP-D complex can deform into the PT pore, by which the inner membrane becomes permeabilized to low _M_r solutes.
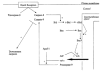
Figure 2. A model for the involvement of the PT pore in necrotic and apoptotic cell death during ischaemia and reperfusion
Ischaemia leads to ATP dissipation, with consequent rises in cell Ca2+ and Pi. On reperfusion, these three factors, together with oxidative stress, trigger PT pore opening. If widespread, PT pore opening overwhelms the ATP-generating capacity of the cell, and the cells become necrotic. If PT pore opening is more limited, enabling the cell to maintain sufficient ATP for viability, then outer membrane rupture may spark a caspase cascade, leading to apoptotis. The degree of PT pore opening may determine the balance between necrotic and apoptotic cell death.
Figure 3. The mitochondrial amplificatory loop
Mitochondria can be recruited into the apoptotic signalling pathway by Bid and Bax, which translocate from the cytosol to the mitochondrial outer membrane under apoptotic stimuli. Bid translocates as a truncated form (t-Bid) after cleavage by caspase-8. Bax is processed on the outer membrane to an active form (Bax*), a process possibly facilitated by t-Bid. Bax induces the release of apoptosis-inducing factor (AIF), procaspase-9, cytochrome c (cyt c) and other intermembrane space proteins to the cytosol. Cytochrome c binds to the adaptor protein Apaf-1, which is then able to bind procaspase-9 and -8, which are thus cleaved to their active forms.
Similar articles
- On the involvement of mitochondrial intermembrane junctional complexes in apoptosis.
Crompton M. Crompton M. Curr Med Chem. 2003 Aug;10(16):1473-84. doi: 10.2174/0929867033457197. Curr Med Chem. 2003. PMID: 12871121 Review. - The mitochondrial permeability transition pore and its role in cell death.
Crompton M. Crompton M. Biochem J. 1999 Jul 15;341 ( Pt 2)(Pt 2):233-49. Biochem J. 1999. PMID: 10393078 Free PMC article. Review. - Mitochondrial intermembrane junctional complexes and their involvement in cell death.
Crompton M, Barksby E, Johnson N, Capano M. Crompton M, et al. Biochimie. 2002 Feb-Mar;84(2-3):143-52. doi: 10.1016/s0300-9084(02)01368-8. Biochimie. 2002. PMID: 12022945 Review. - A mechanistic view of mitochondrial death decision pores.
Belizário JE, Alves J, Occhiucci JM, Garay-Malpartida M, Sesso A. Belizário JE, et al. Braz J Med Biol Res. 2007 Aug;40(8):1011-24. doi: 10.1590/s0100-879x2006005000109. Braz J Med Biol Res. 2007. PMID: 17665037 Review. - Mitochondria and cell death.
Halestrap AP, Doran E, Gillespie JP, O'Toole A. Halestrap AP, et al. Biochem Soc Trans. 2000 Feb;28(2):170-7. doi: 10.1042/bst0280170. Biochem Soc Trans. 2000. PMID: 10816121 Review.
Cited by
- Protective effects of hydroxysafflor yellow A on β-amyloid-induced neurotoxicity in PC12 cells.
Kong SZ, Xian YF, Ip SP, Lai XP, Shi XG, Lin ZX, Su ZR. Kong SZ, et al. Neurochem Res. 2013 May;38(5):951-60. doi: 10.1007/s11064-013-1002-7. Epub 2013 Feb 19. Neurochem Res. 2013. PMID: 23420419 - Ischemic postconditioning at the initiation of cardiopulmonary resuscitation facilitates functional cardiac and cerebral recovery after prolonged untreated ventricular fibrillation.
Segal N, Matsuura T, Caldwell E, Sarraf M, McKnite S, Zviman M, Aufderheide TP, Halperin HR, Lurie KG, Yannopoulos D. Segal N, et al. Resuscitation. 2012 Nov;83(11):1397-403. doi: 10.1016/j.resuscitation.2012.04.005. Epub 2012 Apr 18. Resuscitation. 2012. PMID: 22521449 Free PMC article. - Lactate dehydrogenase is not a mitochondrial enzyme in human and mouse vastus lateralis muscle.
Rasmussen HN, van Hall G, Rasmussen UF. Rasmussen HN, et al. J Physiol. 2002 Jun 1;541(Pt 2):575-80. doi: 10.1113/jphysiol.2002.019216. J Physiol. 2002. PMID: 12042361 Free PMC article. - Biphasic translocation of Bax to mitochondria.
Capano M, Crompton M. Capano M, et al. Biochem J. 2002 Oct 1;367(Pt 1):169-78. doi: 10.1042/BJ20020805. Biochem J. 2002. PMID: 12097139 Free PMC article. - The relationship between intracellular [Ca(2+)] and Ca(2+) wave characteristics in permeabilised cardiomyocytes from the rabbit.
Loughrey CM, MacEachern KE, Neary P, Smith GL. Loughrey CM, et al. J Physiol. 2002 Sep 15;543(Pt 3):859-70. doi: 10.1113/jphysiol.2002.021519. J Physiol. 2002. PMID: 12231644 Free PMC article.
References
- Andreeva L, Tanveer A, Crompton M. Evidence for the involvement of a mitochondrial cyclosporin A binding protein in the Ca-activated inner membrane pore of heart mitochondria. European Journal of Biochemistry. 1995;230:1125–1132. - PubMed
- Ankarcrona M, Dypbukt JM, Bonfoco E, Zhivotovsky B, Orrenius S, Lipton SA, Nicotera P. Glutamate-induced neuronal death: a succession of necrosis or apoptosis depending on mitochondrial function. Neuron. 1995;15:961–973. - PubMed
- Anversa P, Cheng W, Liu Y, Leri A, Redaelli G, Kajstura J. Apoptosis and myocardial infarction. Basic Research in Cardiolology. 1998;93(suppl 3):8–12. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials