Mitochondria and calcium: from cell signalling to cell death - PubMed (original) (raw)
Review
Mitochondria and calcium: from cell signalling to cell death
M R Duchen. J Physiol. 2000.
Abstract
While a pathway for Ca2+ accumulation into mitochondria has long been established, its functional significance is only now becoming clear in relation to cell physiology and pathophysiology. The observation that mitochondria take up Ca2+ during physiological Ca2+ signalling in a variety of cell types leads to four questions: (i) 'What is the impact of mitochondrial Ca2+ uptake on mitochondrial function?' (ii) 'What is the impact of mitochondrial Ca2+ uptake on Ca2+ signalling?' (iii) 'What are the consequences of impaired mitochondrial Ca2+ uptake for cell function?' and finally (iv) 'What are the consequences of pathological [Ca2+]c signalling for mitochondrial function?' These will be addressed in turn. Thus: (i) accumulation of Ca2+ into mitochondria regulates mitochondrial metabolism and causes a transient depolarisation of mitochondrial membrane potential. (ii) Mitochondria may act as a spatial Ca2+ buffer in many cells, regulating the local Ca2+ concentration in cellular microdomains. This process regulates processes dependent on local cytoplasmic Ca2+ concentration ([Ca2+]c), particularly the flux of Ca2+ through IP3-gated channels of the endoplasmic reticulum (ER) and the channels mediating capacitative Ca2+ influx through the plasma membrane. Consequently, mitochondrial Ca2+ uptake plays a substantial role in shaping [Ca2+]c signals in many cell types. (iii) Impaired mitochondrial Ca2+ uptake alters the spatiotemporal characteristics of cellular [Ca2+]c signalling and downregulates mitochondrial metabolism. (iv) Under pathological conditions of cellular [Ca2+]c overload, particularly in association with oxidative stress, mitochondrial Ca2+ uptake may trigger pathological states that lead to cell death. In the model of glutamate excitotoxicity, microdomains of [Ca2+]c are apparently central, as the pathway to cell death seems to require the local activation of neuronal nitric oxide synthase (nNOS), itself held by scaffolding proteins in close association with the NMDA receptor. Mitochondrial Ca2+ uptake in combination with NO production triggers the collapse of mitochondrial membrane potential, culminating in delayed cell death.
Figures
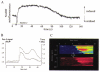
Figure 1. Consequences of mitochondrial Ca2+ uptake for mitochondrial function
A, changes in NADH autofluorescence, excited at 350 nm and recorded at 450 nm, are shown following a 100 ms pulse of 50 m
m
KCl, which was used to depolarise a mouse sensory neuron and thence to raise [Ca2+]c. The autofluorescence initially showed a transient decrease attributable to a transient depolarisation of ΔΨm that accompanies the Ca2+ flux into mitochondria (see below and Fig. 4). This was then superseded by a prolonged increase in signal (increased NADH/NAD+ ratio) which is attributed to activation of the dehydrogenases of the TCA cycle by a high intramitochondrial [Ca2+]. The entire response was blocked by microinjection of the cell with Ruthenium Red (not shown). B, a rise in [Ca2+]c causes a transient mitochondrial depolarisation. Rat cortical astrocytes were loaded with tetramethyl-rhodamine ethyl ester (TMRE) and the [Ca2+]c indicator fluo-3, and imaged simultaneously on a confocal microscope (Zeiss 510CLSM). Application of ATP to a single cell raised [Ca2+]c (•) by IP3-dependent mobilisation from ER stores, and caused a small transient mitochondrial depolarisation (○), signalling mitochondrial Ca2+ uptake (an increase in TMRE fluorescence signals mitochondrial depolarisation – see Boitier et al. 1999). The same phenomenon is seen on a larger scale as [Ca2+]c waves are propagated through a network of interconnected astrocytes, as shown in C. Here, ATP application initiated a wave that propagated from cell to cell, imaged as described above. The steps from cell to cell are indicated by arrows. The simultaneous measurement of ΔΨm reveals a wave of mitochondrial depolarisation propagating through the network.
Figure 4. Effects of toxic glutamate exposure on mitochondrial membrane potential in hippocampal neurons
Changes in ΔΨm in rat hippocampal neurons during changes in [Ca2+]c caused by the prolonged application of 100 μ
m
glutamate (A), 50 m
m
KCl (B), 100 μ
m
glutamate in the presence of the NOS inhibitor L-NAME (100 μ
m
; C) and in the presence of cyclosporin A (CsA, 200 nM; D). In each case, records were obtained using rat hippocampal neurons in culture. Fluorescence signals were recorded from cells loaded with both rhodamine 123 and fura-2 (or its low affinity equivalent fura-2FF) to measure mitochondrial membrane potential and [Ca2+]c simultaneously from a field of 20-30 cells. An increase in the rhodamine 123 signal represents a depolarisation of the mitochondrial membrane (see Keelan et al. 1999), as indicated by the response to FCCP shown at the end of each experiment. The rhodamine signals were normalised between baseline as 0, and the full depolarisation with FCCP as 1.0. In each case, the early changes in [Ca2+]c were not significantly different for any of these manipulations.
Figure 2. Impact of mitochondrial Ca2+ uptake on the propagation of [Ca2+]c waves in rat cortical astrocytes
A, brief application of a threshold concentration of ATP to an adult astrocyte in culture caused a wave of [Ca2+]c which propagated across the cell. The cell was loaded with the [Ca2+]c indicator fluo-3 and imaged using a fast readout cooled CCD camera (Hamamatsu 4880). Plots of intensity with time are shown in _A_i for successive points in space at ≈10 μm intervals across the cell as indicated by the inset. It should be clear that both the rate of propagation and the rate of rise of the [Ca2+]c signal attenuate progressively as the wave progresses along the cell. The mean rate of propagation of the signal was 25 μm s−1. The progress of the wavefront is also illustrated in _A_ii which shows the intensity profile along a line selected along the long axis of the cell with successive image frames from a sequence of just 6 s. The image sequence was first differentiated so that signal is seen only in those pixels in which the signal has changed, and therefore shows the wavefront only. The same principles were applied after depolarisation of ΔΨm in order to limit mitochondrial Ca2+ uptake and data are shown in B i and ii. Now the [Ca2+]c signal clearly propagated faster, with no loss of momentum as it progressed across the cell, and the rate of rise of the signal was sustained throughout the progression of the wave. The mean rate of propagation was now 40 μm s−1.
Figure 3. Scheme to illustrate the interplay between mitochondria and ER [Ca2+]c signalling
These cartoons show a scheme to account for the modulation of [Ca2+]c signalling by mitochondrial Ca2+ uptake in inexcitable cells. The local removal of Ca2+ seems able to buffer [Ca2+]c in local microdomains close to the ER Ca2+ release channels. As IP3-regulated channels are themselves sensitive to [Ca2+]c, this provides a local regulation of their open probability, which serves to regulate the rate and extent of propagation of the signal. When mitochondrial Ca2+ uptake is disabled, this modulation is removed, local [Ca2+]c will be allowed to rise, and will exert feedback regulation on the IP3 receptor (IP3R). The precise consequence may vary depending on the class of IP3 receptor expressed by the cell.
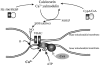
Figure 5. Cartoon to highlight the complex pharmacology of cyclosporin A
Cyclosporin A (CsA) has many actions and cannot be considered specific for the mitochondrial permeability pore. This cartoon illustrates at least two ways in which CsA may interfere with the pathways involved in glutamate excitotoxicity. A local rise in [Ca2+]c through NMDA receptors activates nNOS whose activity is itself regulated through phosphorylation by calcineurin. CsA (and FKBP, the FK-506 binding protein) inhibit the activation of NOS through calcineurin, and, as NO is required for the glutamate-induced mitochondrial depolarisation, CsA may prevent the collapse of ΔΨm through mechanisms quite unrelated to the mPTP. The mPTP itself probably consists of the adenine nucleotide translocase (ANT) and possibly also the voltage-dependent anion channel (VDAC), which form a pore under conditions in which SH groups are oxidised and when intramitochondrial [Ca2+] is high. The pore is regulated by cyclophilin D (CypD), which binds CsA. Therefore CsA may also prevent mitochondrial depolarisation by acting to prevent mPTP opening.
Similar articles
- Flirting in little space: the ER/mitochondria Ca2+ liaison.
Rizzuto R, Duchen MR, Pozzan T. Rizzuto R, et al. Sci STKE. 2004 Jan 13;2004(215):re1. doi: 10.1126/stke.2152004re1. Sci STKE. 2004. PMID: 14722345 Review. - Mitochondrial calcium signalling: message of life and death.
Zecchini E, Siviero R, Giorgi C, Rizzuto R, Pinton P. Zecchini E, et al. Ital J Biochem. 2007 Dec;56(4):235-42. Ital J Biochem. 2007. PMID: 19192620 Review. - Mitochondria and Ca(2+)in cell physiology and pathophysiology.
Duchen MR. Duchen MR. Cell Calcium. 2000 Nov-Dec;28(5-6):339-48. doi: 10.1054/ceca.2000.0170. Cell Calcium. 2000. PMID: 11115373 Review. - Mitochondria as all-round players of the calcium game.
Rizzuto R, Bernardi P, Pozzan T. Rizzuto R, et al. J Physiol. 2000 Nov 15;529 Pt 1(Pt 1):37-47. doi: 10.1111/j.1469-7793.2000.00037.x. J Physiol. 2000. PMID: 11080249 Free PMC article. Review. - The machinery of local Ca2+ signalling between sarco-endoplasmic reticulum and mitochondria.
Hajnóczky G, Csordás G, Madesh M, Pacher P. Hajnóczky G, et al. J Physiol. 2000 Nov 15;529 Pt 1(Pt 1):69-81. doi: 10.1111/j.1469-7793.2000.00069.x. J Physiol. 2000. PMID: 11080252 Free PMC article. Review.
Cited by
- Quantitative, real-time imaging of spreading depolarization-associated neuronal ROS production.
Ackermann MA, Buchholz SM, Dietrich K, Müller M. Ackermann MA, et al. Front Cell Neurosci. 2024 Oct 11;18:1465531. doi: 10.3389/fncel.2024.1465531. eCollection 2024. Front Cell Neurosci. 2024. PMID: 39473491 Free PMC article. - Mitochondrial Impairment: A Link for Inflammatory Responses Activation in the Cardiorenal Syndrome Type 4.
Amador-Martínez I, Aparicio-Trejo OE, Bernabe-Yepes B, Aranda-Rivera AK, Cruz-Gregorio A, Sánchez-Lozada LG, Pedraza-Chaverri J, Tapia E. Amador-Martínez I, et al. Int J Mol Sci. 2023 Nov 1;24(21):15875. doi: 10.3390/ijms242115875. Int J Mol Sci. 2023. PMID: 37958859 Free PMC article. Review. - Gliclazide may have an antiapoptotic effect related to its antioxidant properties in human normal and cancer cells.
Sliwinska A, Rogalska A, Szwed M, Kasznicki J, Jozwiak Z, Drzewoski J. Sliwinska A, et al. Mol Biol Rep. 2012 May;39(5):5253-67. doi: 10.1007/s11033-011-1323-z. Epub 2011 Dec 20. Mol Biol Rep. 2012. PMID: 22183301 Free PMC article. - Ca(2+)N It Be Measured? Detection of Extramitochondrial Calcium Movement With High-Resolution FluoRespirometry.
Nászai A, Terhes E, Kaszaki J, Boros M, Juhász L. Nászai A, et al. Sci Rep. 2019 Dec 17;9(1):19229. doi: 10.1038/s41598-019-55618-5. Sci Rep. 2019. PMID: 31848391 Free PMC article. - Aiming for the target: Mitochondrial drug delivery in traumatic brain injury.
Lamade AM, Kenny EM, Anthonymuthu TS, Soysal E, Clark RSB, Kagan VE, Bayır H. Lamade AM, et al. Neuropharmacology. 2019 Feb;145(Pt B):209-219. doi: 10.1016/j.neuropharm.2018.07.014. Epub 2018 Jul 30. Neuropharmacology. 2019. PMID: 30009835 Free PMC article. Review.
References
- Ankarcrona M, Dypbukt JM, Orrenius S, Nicotera P. Calcineurin and mitochondrial function in glutamate-induced neuronal cell death. FEBS Letters. 1996;394:321–324. - PubMed
- Bezprozvanny I, Watras J, Ehrlich BE. Bell-shaped calcium-response curves of Ins(1,4,5)P3- and calcium-gated channels from endoplasmic reticulum of cerebellum. Nature. 1991;351:751–754. - PubMed
- Brini M, Marsault R, Bastianutto C, Alvarez J, Pozzan T, Rizzuto R. Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]c). A critical evaluation. Journal of Biological Chemistry. 1995;270:9896–9903. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous