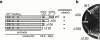Nmd3p is a Crm1p-dependent adapter protein for nuclear export of the large ribosomal subunit - PubMed (original) (raw)
Nmd3p is a Crm1p-dependent adapter protein for nuclear export of the large ribosomal subunit
J H Ho et al. J Cell Biol. 2000.
Abstract
In eukaryotic cells, nuclear export of nascent ribosomal subunits through the nuclear pore complex depends on the small GTPase Ran. However, neither the nuclear export signals (NESs) for the ribosomal subunits nor the receptor proteins, which recognize the NESs and mediate export of the subunits, have been identified. We showed previously that Nmd3p is an essential protein from yeast that is required for a late step in biogenesis of the large (60S) ribosomal subunit. Here, we show that Nmd3p shuttles and that deletion of the NES from Nmd3p leads to nuclear accumulation of the mutant protein, inhibition of the 60S subunit biogenesis, and inhibition of the nuclear export of 60S subunits. Moreover, the 60S subunits that accumulate in the nucleus can be coimmunoprecipitated with the NES-deficient Nmd3p. 60S subunit biogenesis and export of truncated Nmd3p were restored by the addition of an exogenous NES. To identify the export receptor for Nmd3p we show that Nmd3p shuttling and 60S export is blocked by the Crm1p-specific inhibitor leptomycin B. These results identify Crm1p as the receptor for Nmd3p export. Thus, export of the 60S subunit is mediated by the adapter protein Nmd3p in a Crm1p-dependent pathway.
Figures
Figure 1
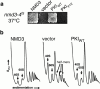
Phenotype of Nmd3p COOH-terminal truncation mutants. (a) A diagram of Nmd3p wild-type and deletion mutants showing the amino-terminal domain conserved in archaebacterial and eukaryotic proteins (gray) containing four Zn2+-binding motifs (cys-x2-cys), the basic cluster (aa 399–419, stippled), and the acidic COOH terminus (aa 476–518, hatched). NLS and NES indicate approximate positions of NLS and NES, respectively. Numbers indicate aa positions. Complementation was assayed by plasmid shuffle and indicates the ability to replace a plasmid-borne wild-type NMD3 in an nmd3::TRP1 disruption strain. (b) The proteins depicted in panel a were tagged with the c-myc epitope and expressed from centromeric vectors in the wild-type yeast strain CH1305 (Kranz and Holm 1990). Transformants were streaked for single colonies on a selective plate and incubated for 2 d at 30°C.
Figure 2
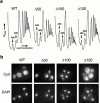
Dominant Nmd3p truncation mutants inhibit 60S biogenesis and are localized to the nucleus. (a) Extracts, prepared from cultures of the transformants shown in Fig. 1 b, were fractionated by ultracentrifugation in 7–47% sucrose gradients, as described previously (Ho et al. 2000). The positions of free 40S and 60S subunits are indicated. The relative peak heights of free subunits are a sensitive indicator of total subunit levels and, hence, biogenesis defects. (b) Indirect immunofluorescence of the c-myc–tagged proteins in yeast. Cy3, rhodamine channel for c-myc–tagged proteins; DAPI, UV channel indicating the position of nuclei.
Figure 5
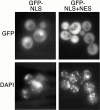
Localization of GFP directed by NLS and NES of Nmd3p. The putative NLS of Nmd3p (aa 387–435) and the NLS plus the NES (aa 387–518) were fused to GFP and introduced into wild-type cells (CH1305). The localization of the GFP fusion proteins was determined by direct fluorescence (GFP), and DNA was stained with DAPI in living cells. The multiple small DAPI-stained spots are mitochondrial DNA.
Figure 3
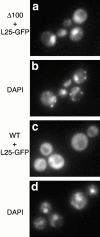
Nmd3Δ100 traps 60S ribosomal subunits in the nucleus. Galactose-inducible L25–GFP and Nmd3Δ100 (13-myc–tagged) or full-length Nmd3p were introduced into wild-type cells (CH1305) on plasmids. Cultures were grown in the presence of raffinose and induced by the addition of galactose. DAPI was added at this time to 2.5 μg/ml. 3 h after induction, the localization of (a and c) L25–GFP or (b and d) DAPI was visualized by fluorescence microscopy. (a and b) Cells coexpressing Nmd3Δ100 and L25–GFP. (c and d) Cells coexpressing full-length Nmd3p and L25–GFP. Similar results were obtained with Nmd3Δ100 without epitope tag (data not shown).
Figure 4
Nmd3Δ100 coimmunoprecipitates 60S subunits containing L25–GFP. (a) Extracts were prepared from cells coexpressing L25–GFP and Nmd3Δ100 with 13-myc (+) or Nmd3Δ100 without 13-myc tag (−). The same amount of total protein from each extract was used. Immunoprecipitation was carried out by the addition of anti–c-myc antibody and protein A beads, and the immunoprecipitated samples were analyzed by SDS-PAGE and Western blot analyisis. Nmd3, Nmd3Δ100; GFP, L25–GFP; L12, wild-type 60S subunit protein L12. (b) Gel-separated proteins from panel a were stained with Coomassie blue. Nmd3Δ100, immunoglobulin heavy and light chains (IgG), and ribosomal proteins (asterisks) are indicated. The identity of the low molecular weight proteins as 60S subunit proteins was confirmed by direct comparison with purified 60S subunits by SDS-PAGE and by Western blot analysis with multiple antibodies specific for 60S proteins (data not shown).
Figure 7
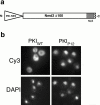
Rescue of Nmd3Δ100 function with a heterologous NES. (a) Plasmids expressing wild-type Nmd3p (NMD3), no protein (vector), and Nmd3Δ100 containing the PKIWT or mutant PKIP12 NES were introduced into the temperature-sensitive nmd3-4 mutant. Transformants were patched onto selective medium and grown for 2 d at 37°C. Similar results were obtained in an nmd3::TRP1 disruption strain (data not shown). The dominant-negative effect of Nmd3Δ100 containing the mutant NES (PKIP12) severely impaired cell growth even at permissive temperature, leading to a phenotype more severe than that of nmd3-4 cells containing an empty vector. (b) Sucrose gradient analysis was performed. Cultures of the transformants shown in panel a were grown at permissive temperature, shifted to 37°C for 3 h, and the status of ribosomal subunits and polysomes was analyzed by sucrose gradient sedimentation, as described previously (Ho et al. 2000). Nmd3Δ100 containing the mutant NES (PKIP12) was not analyzed due to its severe growth defect. The positions of free 40S, free 60S, and halfmers (polysomes containing an unjoined 40S subunit at the initiation site resulting from the deficit of free 60S subunits) are shown.
Figure 6
The addition of a functional NES to Nmd3Δ100 relocalizes the protein to the cytoplasm. (a) A cartoon is shown of the addition of a heterologous NES to the NH2 terminus of Nmd3Δ100. (b) The fusion proteins were expressed from centromeric plasmids in the wild-type strain (CH1305). The localization of the proteins was determined by indirect immunofluorescence (Cy3), and DNA was stained with DAPI.
Figure 8
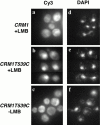
Nmd3p localization is sensitive to the Crm1p inhibitor leptomycin B. (a and d) The wild-type strain MNY7 (CRM1) and (b, c, e, and f) the leptomycin-sensitive strain MNY8 (CRM1T539C) were transformed with plasmid pAJ412 expressing c-myc–tagged Nmd3p. Cells were grown to early log phase and treated (a, b, d, and e) with (+LMB) leptomycin B (0.1 μg/ml) or (c and f) as controls without (−LMB) leptomycin B and incubated for 15 min. Cells were then fixed and prepared for indirect immunofluorescence. Cy3, rhodamine channel for c-myc–tagged Nmd3p; DAPI, UV channel for DNA. The localization of Nmd3p in CRM1 cells, in the absence of leptomycin B, was similar to CRM1 cells in the presence of leptomycin B (data not shown).
Figure 9
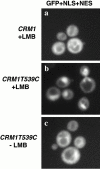
GFP containing the NLS and NES of Nmd3p is restricted to the nucleus, in the presence of leptomycin B. (a) MNY7 (CRM1) and the (b and c) leptomycin-sensitive strain MNY8 (CRM1T539C) cells were transformed with plasmid pAJ629 expressing galactose-inducible GFP fused to the NLS plus NES of Nmd3p (aa 387–518). Cells were grown to early log phase and galactose was added to a final concentration of 1% to induce GFP expression. After 1 h of induction, leptomycin B was added to 0.1 μg/ml (+LMB). (c) For controls, leptomycin B was omitted (−LMB). GFP was visualized by direct fluorescence microscopy 1 h after the addition of leptomycin B. GFP localization to the nucleus was evident within 15 min of the addition of leptomycin (data not shown).
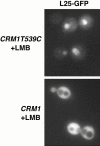
Figure 10
L25–GFP is retained in the nucleus in the presence of leptomycin B. (a) The leptomycin-sensitive strain MNY8 (CRM1T539C) and (b) the wild-type strain MNY7 (CRM1) were transformed with plasmid pAJ369, which expresses L25–GFP. Overnight cultures grown in medium lacking uracil, with raffinose as the carbon source, were diluted into the same medium and grown for 4 h at 30°C. Leptomycin B and galactose were then added to 0.1 μg/ml and 1%, respectively. After 4 h of incubation at 30°C, GFP fluorescence was visualized. In the absence of added leptomycin B, L25–GFP was cytoplasmic in both strains (data not shown).
Similar articles
- Nuclear export of 60s ribosomal subunits depends on Xpo1p and requires a nuclear export sequence-containing factor, Nmd3p, that associates with the large subunit protein Rpl10p.
Gadal O, Strauss D, Kessl J, Trumpower B, Tollervey D, Hurt E. Gadal O, et al. Mol Cell Biol. 2001 May;21(10):3405-15. doi: 10.1128/MCB.21.10.3405-3415.2001. Mol Cell Biol. 2001. PMID: 11313466 Free PMC article. - The putative GTPases Nog1p and Lsg1p are required for 60S ribosomal subunit biogenesis and are localized to the nucleus and cytoplasm, respectively.
Kallstrom G, Hedges J, Johnson A. Kallstrom G, et al. Mol Cell Biol. 2003 Jun;23(12):4344-55. doi: 10.1128/MCB.23.12.4344-4355.2003. Mol Cell Biol. 2003. PMID: 12773575 Free PMC article. - Nascent 60S ribosomal subunits enter the free pool bound by Nmd3p.
Ho JH, Kallstrom G, Johnson AW. Ho JH, et al. RNA. 2000 Nov;6(11):1625-34. doi: 10.1017/s1355838200001291. RNA. 2000. PMID: 11105761 Free PMC article. - Nuclear export of ribosomal subunits.
Johnson AW, Lund E, Dahlberg J. Johnson AW, et al. Trends Biochem Sci. 2002 Nov;27(11):580-5. doi: 10.1016/s0968-0004(02)02208-9. Trends Biochem Sci. 2002. PMID: 12417134 Review. - Nuclear export and cytoplasmic maturation of ribosomal subunits.
Zemp I, Kutay U. Zemp I, et al. FEBS Lett. 2007 Jun 19;581(15):2783-93. doi: 10.1016/j.febslet.2007.05.013. Epub 2007 May 11. FEBS Lett. 2007. PMID: 17509569 Review.
Cited by
- Strength in Diversity: Nuclear Export of Viral RNAs.
Gales JP, Kubina J, Geldreich A, Dimitrova M. Gales JP, et al. Viruses. 2020 Sep 11;12(9):1014. doi: 10.3390/v12091014. Viruses. 2020. PMID: 32932882 Free PMC article. Review. - The yeast nuclear pore complex and transport through it.
Aitchison JD, Rout MP. Aitchison JD, et al. Genetics. 2012 Mar;190(3):855-83. doi: 10.1534/genetics.111.127803. Genetics. 2012. PMID: 22419078 Free PMC article. - Non-FG mediated transport of the large pre-ribosomal subunit through the nuclear pore complex by the mRNA export factor Gle2.
Occhipinti L, Chang Y, Altvater M, Menet AM, Kemmler S, Panse VG. Occhipinti L, et al. Nucleic Acids Res. 2013 Sep;41(17):8266-79. doi: 10.1093/nar/gkt675. Epub 2013 Jul 31. Nucleic Acids Res. 2013. PMID: 23907389 Free PMC article. - NESdb: a database of NES-containing CRM1 cargoes.
Xu D, Grishin NV, Chook YM. Xu D, et al. Mol Biol Cell. 2012 Sep;23(18):3673-6. doi: 10.1091/mbc.E12-01-0045. Epub 2012 Jul 25. Mol Biol Cell. 2012. PMID: 22833564 Free PMC article. - Comparative genomics of proteins involved in RNA nucleocytoplasmic export.
Serpeloni M, Vidal NM, Goldenberg S, Avila AR, Hoffmann FG. Serpeloni M, et al. BMC Evol Biol. 2011 Jan 11;11:7. doi: 10.1186/1471-2148-11-7. BMC Evol Biol. 2011. PMID: 21223572 Free PMC article.
References
- Caponigro G., Parker R. Multiple functions for the poly(A)-binding protein in mRNA decapping and deadenylation in yeast. Genes Dev. 1995;9:2421–2432. - PubMed
- Fornerod M., Ohno M., Yoshida M., Mattaj I.W. CRM1 is an export receptor for leucine-rich nuclear export signals. Cell. 1997;90:1051–1060. - PubMed
- Görlich D., Kutay U. Transport between the cell nucleus and the cytoplasm. Annu. Rev. Cell. Dev. Biol. 1999;15:607–660. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous