CD8(+) T-cell selection, function, and death in the primary immune response in vivo - PubMed (original) (raw)
CD8(+) T-cell selection, function, and death in the primary immune response in vivo
M F Callan et al. J Clin Invest. 2000 Nov.
Abstract
The primary immune response to Epstein Barr virus (EBV) is characterized by striking proliferation of EBV-specific CD8(+) T cells. In this study we have investigated the clonal composition and functional properties of the cells mediating this primary response and have analyzed the mechanisms that control the downregulation of the primary response and the selection of memory cells. We show that massively expanded T-cell clones often dominate the primary antigen-specific T-cell response. Despite the enormous extent of expansion, the virus-specific T cells express high levels of intracellular perforin and are potently cytotoxic. They are, however, functionally heterogeneous in their ability to secrete proinflammatory cytokines, with subpopulations of the antigen-specific T cells being hyporesponsive. The primary response is closely regulated, and the majority of cells are programmed to die via a cytokine-rescuable pathway, leaving only small populations of memory T cells surviving. Comparison of the clonal composition of primary and memory responses in vivo shows that the clones that dominate the primary response are relatively heavily culled during the downregulation of the primary response and the establishment of T-cell memory.
Figures
Figure 1
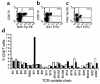
BV7S1 use by HLA-B8–restricted RAKFKQLL-specific T cells in patient NS21 during the primary response to EBV. PBMCs taken from patient NS21 during primary EBV infection were stained with TCR V region–specific mAb’s, with an anti-CD8 mAb, and with the B8/RAKFKQLL tetrameric complex. (a) Twenty-eight percent of the CD8+ T cells react with the B8/RAKFKQLL tetrameric complex; (b) 18% of CD8+ T cells express the TCR BV7S1 chain; (c) 57% of B8/RAKFKQLL tetramer–reactive T cells express BV7S1. (d) The overall CD8+ TCR repertoire of patient NS21 (filled bars) is distorted with an expanded population of CD8+ T cells expressing BV7S1. The mean TCR repertoire of a panel of 21 young control individuals is shown (open bars) for comparison.
Figure 2
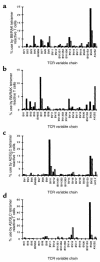
The TCR repertoire of CD8+ T cells specific for EBV antigens during the primary and memory immune responses. PBMCs taken from donor NS52 (a), donor NS60 (b), donor NS33 (c), and donor NS60 (d), during primary EBV infection (black bars) and 1 year later (hatched bars), were stained with a panel of TCR V region–specific mAb’s, with the B8/RAKFKQLL tetrameric complex (a and b), or with the A2/GLCTLVAML tetrameric complex (c and d), and with an Ab specific for CD8. The TCR V–region use of CD8+ T cells reacting with the relevant tetrameric complex is shown. The frequency (% of CD8+ T cells) of RAKFKQLL-specific T cells during primary infection and 1 year later in donor NS52 was 40.2% and 4.4%, respectively, and in donor NS60 was 33% and 4.3%, respectively. The frequency of GLCTLVAML-specific T cells during primary infection and 1 year later in donor NS33 was 11% and 5.5%, respectively, and in donor NS60 was 6.6% and 1.3%, respectively.
Figure 3
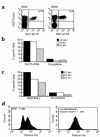
Cytotoxicity of EBV-specific T cells during the primary immune response. (a) PBMCs from donor NS30, an HLA-A2+ individual with IM, and donor NS28, an HLA-B8+ individual with IM, were stained with an A2/GLCTLVAML or a B8/RAKFKQLL tetrameric complex and with an anti-CD8 mAb. The frequency (%) of CD8+ T cells that stain with the tetrameric complex is shown. PBMCs from these two patients were used as effector cells in chromium-release assays. PBMCs from donor NS30 caused specific lysis of target cells prepulsed with the GLCTLVAML peptide (b), and PBMCs from donor NS28 caused specific lysis of the targets prepulsed with the RAKFKQLL peptide (c). PBMCs from the HLA-B8+ donor were stained with the B8/RAKFKQLL tetrameric complex, with anti-CD8, and then were fixed, permeabilized, and stained for intracellular perforin expression. Perforin expression on CD8+ T cells was bimodal with 57% of CD8+ T cells being perforin bright. All the HLA-B8/RAKFKQLL tetramer–reactive T cells were perforin bright. The FL-1 threshold was set using a FITC-conjugated IgG2b control mAb.
Figure 4
Cytokine secretion by EBV-specific T cells during the primary immune response. PBMCs from HLA-A2+ individuals with IM (NS120 and NS122) were stained with the HLA-A2/GLCTLVAML tetrameric complex. PBMCs from NS120 were then cultured in vitro for 6 hours in the absence (a and b) or the presence (c and d) of 10 μM GLCTLVAML peptide in R10 supplemented with rIL-2. PBMCs from NS122 were cultured in vitro for 6 hours in the absence (e and f) or presence (g and h) of an autologous lymphoblastoid B-cell line (at a ratio of 1 B cell/1 PBMC) prepulsed with 10 μM GLCTLVAML peptide in R10 supplemented with rIL-2. Brefeldin A was added to the cultures after the first hour. Cells were surface-stained with an anti-CD8 mAb, fixed, permeabilized, and stained for intracellular IFN-γ (a, c, e, and g) or MIP1 β (b, d, f, and h). Only CD8+ T cells were included in the analyses. The frequency (%) of tetramer-reactive T cells that stained positively with mAb’s specific for IFN-γ or MIP1 β is shown.
Figure 5
Death of antigen-specific T cells taken from patients with primary EBV infection. (a) PBMCs from HLA-A2+ (NS80, NS81, NS97) and HLA-B8+ (NS51, NS100) individuals with IM and from HLA-A2+ (C1, C3, C4) and HLA-B8+ (C2, C5) healthy EBV-seropositive individuals were cultured for 30 hours in R10. The frequency of live CD8+ T cells that reacted with the HLA-A2/GLCTLVAML or HLA-B8/RAKFKQLL tetrameric complexes as a proportion of the total lymphocytes was calculated at 0 hours and 30 hours. Survival (%) of the tetramer-reactive T cells at 30 hours is shown. (b) PBMCs from an HLA-A2+ (NS80) and HLA-B8+ (NS51) individual with IM were cultured in R10, in R10 in the presence of 100 ng/ml Fas-Fc fusion protein, or in R10 in the presence of 500 ng/ml DR5-Fc fusion protein for 30 hours. Survival (%) of the tetramer-reactive T cells is shown. (c) PBMCs from an HLA-A2+ (NS97) and HLA-B8+ (NS100) individual with IM were cultured in R10, in R10 in the presence of 50 U/ml rIL-2, 100 ng/ml rIL-7 or 100 ng/ml rIL-15. Survival (%) of the tetramer-reactive T cells is shown.
Figure 6
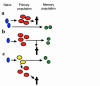
Models for the development of T-cell memory. (a) Primary and memory populations of T cells may derive from different naive precursors. The TCR use of the primary and memory populations would not be closely related. (b) Naive T cells differentiate to form a primary population. A minority of these cells persist into memory, while the majority die. The TCR use of the memory population would be identical to, or possibly more restricted than, that of the primary population. (c) Naive T cells differentiate to form an intermediate core cell from which both an expanded primary population (destined to die) and a memory population may derive. The TCR use of the memory population would be similar to that of the primary population, but relative depletion of the clonotypes most expanded during the primary response might occur.
Similar articles
- Severity of Acute Infectious Mononucleosis Correlates with Cross-Reactive Influenza CD8 T-Cell Receptor Repertoires.
Aslan N, Watkin LB, Gil A, Mishra R, Clark FG, Welsh RM, Ghersi D, Luzuriaga K, Selin LK. Aslan N, et al. mBio. 2017 Dec 5;8(6):e01841-17. doi: 10.1128/mBio.01841-17. mBio. 2017. PMID: 29208744 Free PMC article. - Immediate early and early lytic cycle proteins are frequent targets of the Epstein-Barr virus-induced cytotoxic T cell response.
Steven NM, Annels NE, Kumar A, Leese AM, Kurilla MG, Rickinson AB. Steven NM, et al. J Exp Med. 1997 May 5;185(9):1605-17. doi: 10.1084/jem.185.9.1605. J Exp Med. 1997. PMID: 9151898 Free PMC article. - Detailed kinetics of EBV-specific CD4+ and CD8+ T cells during primary EBV infection in a kidney transplant patient.
Piriou ER, van Dort K, Weel JF, Bemelman FJ, Gamadia LE, van Oers MH, van Baarle D. Piriou ER, et al. Clin Immunol. 2006 Apr;119(1):16-20. doi: 10.1016/j.clim.2005.11.009. Epub 2006 Jan 4. Clin Immunol. 2006. PMID: 16386961 - Immunodominant CD8 T cell response to Epstein-Barr virus.
Houssaint E, Saulquin X, Scotet E, Bonneville M. Houssaint E, et al. Biomed Pharmacother. 2001 Sep;55(7):373-80. doi: 10.1016/s0753-3322(01)00082-8. Biomed Pharmacother. 2001. PMID: 11669500 Review. - Cell-mediated immunity against Epstein-Barr virus infected B lymphocytes.
Klein E, Masucci MG. Klein E, et al. Springer Semin Immunopathol. 1982;5(1):63-73. doi: 10.1007/BF00201957. Springer Semin Immunopathol. 1982. PMID: 6314570 Review. No abstract available.
Cited by
- Alloantigen specific deletion of primary human T cells by Fas ligand (CD95L)-transduced monocyte-derived killer-dendritic cells.
Schütz C, Hoves S, Halbritter D, Zhang HG, Mountz JD, Fleck M. Schütz C, et al. Immunology. 2011 May;133(1):115-22. doi: 10.1111/j.1365-2567.2011.03417.x. Epub 2011 Feb 22. Immunology. 2011. PMID: 21342185 Free PMC article. - Characterization of the CD4+ T cell response to Epstein-Barr virus during primary and persistent infection.
Amyes E, Hatton C, Montamat-Sicotte D, Gudgeon N, Rickinson AB, McMichael AJ, Callan MF. Amyes E, et al. J Exp Med. 2003 Sep 15;198(6):903-11. doi: 10.1084/jem.20022058. J Exp Med. 2003. PMID: 12975456 Free PMC article. - Novel strategies for adoptive therapy following HLA disparate transplants.
O'Reilly RJ, Hasan A, Doubrovina E, Koehne G, Prockop S. O'Reilly RJ, et al. Best Pract Res Clin Haematol. 2011 Sep;24(3):381-91. doi: 10.1016/j.beha.2011.06.001. Best Pract Res Clin Haematol. 2011. PMID: 21925091 Free PMC article. Review. - Assessing CD8 T cell number and dysfunction in the presence of antigen.
Welsh RM. Welsh RM. J Exp Med. 2001 Mar 5;193(5):F19-22. doi: 10.1084/jem.193.5.f19. J Exp Med. 2001. PMID: 11238597 Free PMC article. Review. No abstract available. - CDR3α drives selection of the immunodominant Epstein Barr virus (EBV) BRLF1-specific CD8 T cell receptor repertoire in primary infection.
Kamga L, Gil A, Song I, Brody R, Ghersi D, Aslan N, Stern LJ, Selin LK, Luzuriaga K. Kamga L, et al. PLoS Pathog. 2019 Nov 25;15(11):e1008122. doi: 10.1371/journal.ppat.1008122. eCollection 2019 Nov. PLoS Pathog. 2019. PMID: 31765434 Free PMC article.
References
- Murali-Krishna K, et al. Counting antigen-specific CD8+ T cells: a reevaluation of bystander activation during viral infection. Immunity. 1998;8:177–187. - PubMed
- Tan L, et al. A re-evaluation of the frequency of CD8+ T cells specific for EBV in healthy virus carriers. J Immunol. 1999;162:1827–1835. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials