The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters - PubMed (original) (raw)
The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: peptoid molecular transporters
P A Wender et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Certain proteins contain subunits that enable their active translocation across the plasma membrane into cells. In the specific case of HIV-1, this subunit is the basic domain Tat(49-57) (RKKRRQRRR). To establish the optimal structural requirements for this translocation process, and thereby to develop improved molecular transporters that could deliver agents into cells, a series of analogues of Tat(49-57) were prepared and their cellular uptake into Jurkat cells was determined by flow cytometry. All truncated and alanine-substituted analogues exhibited diminished cellular uptake, suggesting that the cationic residues of Tat(49-57) play a principal role in its uptake. Charge alone, however, is insufficient for transport as oligomers of several cationic amino acids (histidine, lysine, and ornithine) are less effective than Tat(49-57) in cellular uptake. In contrast, a 9-mer of l-arginine (R9) was 20-fold more efficient than Tat(49-57) at cellular uptake as determined by Michaelis-Menton kinetic analysis. The d-arginine oligomer (r9) exhibited an even greater uptake rate enhancement (>100-fold). Collectively, these studies suggest that the guanidinium groups of Tat(49-57) play a greater role in facilitating cellular uptake than either charge or backbone structure. Based on this analysis, we designed and synthesized a class of polyguanidine peptoid derivatives. Remarkably, the subset of peptoid analogues containing a six-methylene spacer between the guanidine head group and backbone (N-hxg), exhibited significantly enhanced cellular uptake compared to Tat(49-57) and even to r9. Overall, a transporter has been developed that is superior to Tat(49-57), protease resistant, and more readily and economically prepared.
Figures
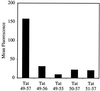
Figure 1
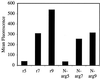
FACS cellular uptake assay of truncated analogs of Tat49–57 (Fl-ahx-RKKRRQRRR): Tat49–56 (Fl-ahx-RKKRRQRR), Tat49–55 (Fl-ahx-RKKRRQR), Tat50–57 (Fl-ahx-KKRRQRRR), and Tat51–57 (Fl-ahx-KRRQRRR). Jurkat cells were incubated with varying concentrations (12.5 μM shown) of peptides for 10 min at 23°C.
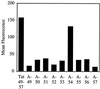
Figure 2
FACS cellular uptake assay of alanine-substituted analogs of Tat49–57: A-49 (Fl-ahx-AKKRRQRRR), A-50 (Fl-ahx-RAKRRQRRR), A-51 (Fl-ahx-RKARRQRRR), A-52 (Fl-ahx-RKKARQRRR), A-53 (Fl-ahx-RKKRAQRRR), A-54 (Fl-ahx-RKKRRARRR), A-55 (Fl-ahx-RKKRRQARR), A-56 (Fl-ahx-RKKRRQRAR), and A-57 (Fl-ahx-RKKRRQRRA). Jurkat cells were incubated with varying concentrations (12.5 μM shown) of peptides for 10 min at 23°C.
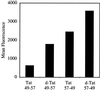
Figure 3
FACS cellular uptake assay of _d_- and retro-isomers of Tat49–57: _d_-Tat49–57 (Fl-ahx-rkkrrqrrr), Tat57–49 (Fl-ahx-RRRQRRKKR), and_d_-Tat57–49 (Fl-ahx-rrrqrrkkr). Jurkat cells were incubated with varying concentrations (12.5 μM shown) of peptides for 15 min at 23°C.
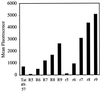
Figure 4
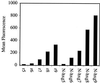
FACS cellular uptake of a series of arginine oligomers and Tat49–57: R5 (Fl-ahx-RRRRR), R6 (Fl-ahx-RRRRRR), R7 (Fl-ahx-RRRRRRR), R8 (Fl-ahx-RRRRRRRR), R9 (Fl-ahx-RRRRRRRRR), r5 (Fl-ahx-rrrrr), r6 (Fl-ahx-rrrrrr), r7 (Fl-ahx-rrrrrrr), r8 (Fl-ahx-rrrrrrrr), r9 (Fl-ahx-rrrrrrrrr). Jurkat cells were incubated with varying concentrations (12.5 μM shown) of peptides for 15 min at 23°C.
Scheme 1
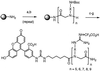
(a) BrCH2CO2H, DIC, DMF. (b) BocNH-X-NH2, DMF. (c) Fmoc-ahx-CO2H, DIC, DMF. (d) piperidine, DMF. (e) FITC-NCS, DIEA, DMF. (f) 95:5 TFA/TIS. (g) pyrazole-1-carboxamidine, aq. Na2CO3, 50°C. Abbreviations: _N_-etg, X = (CH2)2; N-arg, X = (CH2)3; _N_-btg, X = (CH2)4; _N_-hxg, X = (CH2)6; _N_-ocg, X = (CH2)8; _N_-chg, X =_trans_-1,4-cyclohexyl.
Figure 5
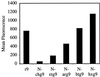
FACS cellular uptake of polyguanidine peptoids and
d
-arginine oligomers. Jurkat cells were incubated with varying concentrations (12.5 μM shown) of peptoids and peptides for 4 min at 23°C.
Figure 6
FACS cellular uptake of
d
-arginine oligomers and polyguanidine peptoids. Jurkat cells were incubated with varying concentrations (12.5 μM shown) of fluorescently labeled peptoids and peptides for 4 min at 23°C.
Figure 7
FACS cellular uptake of
d
-arginine oligomers and_N_-hxg peptoids. Jurkat cells were incubated with varying concentrations (6.3 μM shown) of fluorescently labeled peptoids and peptides for 4 min at 23°C.
Figure 8
FACS cellular uptake of
d
-arginine oligomers and_N_-chg peptoids. Jurkat cells were incubated with varying concentrations (12.5 μM shown) of fluorescently labeled peptoids and peptides for 4 min at 23°C.
Similar articles
- A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus.
Vivès E, Brodin P, Lebleu B. Vivès E, et al. J Biol Chem. 1997 Jun 20;272(25):16010-7. doi: 10.1074/jbc.272.25.16010. J Biol Chem. 1997. PMID: 9188504 - Translocation of branched-chain arginine peptides through cell membranes: flexibility in the spatial disposition of positive charges in membrane-permeable peptides.
Futaki S, Nakase I, Suzuki T, Youjun Z, Sugiura Y. Futaki S, et al. Biochemistry. 2002 Jun 25;41(25):7925-30. doi: 10.1021/bi0256173. Biochemistry. 2002. PMID: 12069581 - Tat peptide-mediated cellular delivery: back to basics.
Brooks H, Lebleu B, Vivès E. Brooks H, et al. Adv Drug Deliv Rev. 2005 Feb 28;57(4):559-77. doi: 10.1016/j.addr.2004.12.001. Epub 2005 Jan 6. Adv Drug Deliv Rev. 2005. PMID: 15722164 Review. - Structural variety of membrane permeable peptides.
Futaki S, Goto S, Suzuki T, Nakase I, Sugiura Y. Futaki S, et al. Curr Protein Pept Sci. 2003 Apr;4(2):87-96. doi: 10.2174/1389203033487261. Curr Protein Pept Sci. 2003. PMID: 12678848 Review.
Cited by
- Comparative study of guanidine-based and lysine-based brush copolymers for plasmid delivery.
Carlson PM, Schellinger JG, Pahang JA, Johnson RN, Pun SH. Carlson PM, et al. Biomater Sci. 2013 Jul 1;1(7):736-744. doi: 10.1039/C3BM60079C. Biomater Sci. 2013. PMID: 23750319 Free PMC article. - Physicochemical Features and Peculiarities of Interaction of AMP with the Membrane.
Pirtskhalava M, Vishnepolsky B, Grigolava M, Managadze G. Pirtskhalava M, et al. Pharmaceuticals (Basel). 2021 May 17;14(5):471. doi: 10.3390/ph14050471. Pharmaceuticals (Basel). 2021. PMID: 34067510 Free PMC article. Review. - Strategies for Improving Peptide Stability and Delivery.
Al Musaimi O, Lombardi L, Williams DR, Albericio F. Al Musaimi O, et al. Pharmaceuticals (Basel). 2022 Oct 19;15(10):1283. doi: 10.3390/ph15101283. Pharmaceuticals (Basel). 2022. PMID: 36297395 Free PMC article. Review. - A noncovalent, fluoroalkyl coating monomer for phosphonate-covered nanoparticles.
Li V, Chang AY, Williams TJ. Li V, et al. Tetrahedron. 2013 Sep 9;69(36):7741-7746. doi: 10.1016/j.tet.2013.05.092. Tetrahedron. 2013. PMID: 23913989 Free PMC article. - Efficient Cargo Delivery into Adult Brain Tissue Using Short Cell-Penetrating Peptides.
Kizil C, Iltzsche A, Thomas AK, Bhattarai P, Zhang Y, Brand M. Kizil C, et al. PLoS One. 2015 Apr 20;10(4):e0124073. doi: 10.1371/journal.pone.0124073. eCollection 2015. PLoS One. 2015. PMID: 25894337 Free PMC article.
References
- Terwogt J M M, Nuijen B, Huinink W W T B, Beignen J H. Cancer Treat Rev. 1997;23:87–95. - PubMed
- Rait A, Pirollo K, Will D W, Peyman A, Rait V, Uhlmann E, Chang E H. Bioconjugate Chem. 2000;11:153–160. - PubMed
- Rui Y J, Wang S, Low P S, Thompson D H. J Am Chem Soc. 1998;120:11213–11218.
- Kono K, Liu M, Fréchet J M J. Bioconjugate Chem. 1999;10:1115–1121. - PubMed
- Ghosh A, Ghosh M, Niu C, Malouin F, Moellmann U, Miller M J. Chem Biol. 1996;3:1011–1019. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R37 CA031841/CA/NCI NIH HHS/United States
- R01 CA031845/CA/NCI NIH HHS/United States
- F32 CA080344/CA/NCI NIH HHS/United States
- AI 40968/AI/NIAID NIH HHS/United States
- CA 31841/CA/NCI NIH HHS/United States
- R01 CA031841/CA/NCI NIH HHS/United States
- CA 31845/CA/NCI NIH HHS/United States
- R37 CA031845/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous