Histone H4 acetylation of euchromatin and heterochromatin is cell cycle dependent and correlated with replication rather than with transcription - PubMed (original) (raw)
Histone H4 acetylation of euchromatin and heterochromatin is cell cycle dependent and correlated with replication rather than with transcription
Z Jasencakova et al. Plant Cell. 2000 Nov.
Abstract
Reversible acetylation of nucleosomal histones H3 and H4 generally is believed to be correlated with potential transcriptional activity of eukaryotic chromatin domains. Here, we report that the extent of H4 acetylation within euchromatin and heterochromatic domains is linked with DNA replication rather than with transcriptional activity, whereas H3 acetylation remains fairly constant throughout the cell cycle. Compared with euchromatin, plant nucleolus organizers were more strongly acetylated at H4 during mitosis but less acetylated during S phase, when the nucleolus appeared to be (at least transiently) devoid of nucleosomes. Deposition-related acetylation of lysines 5 and 12 of H4 seems to be conserved in animals and plants and extended to K16 in plants. A possibly species-specific above-average acetylation at lysines 9/18 and 14 of H3 appeared in 4',6-diamidino-2-phenylindole (DAPI)-stained heterochromatin fractions. These results were obtained by combining immunodetection of all acetylatable isoforms of H3 and H4 on mitotic chromosomes and nuclei in G1, early S, mid-S, late S, and G2 phases of the field bean with identification of specific chromatin domains by fluorescence in situ hybridization or DAPI staining. In addition, the histone acetylation patterns of distinct domains were compared with their replication and transcription patterns.
Figures
Figure 1.
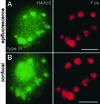
The Six Chromosomes of the Field Bean Karyotype ACB. (A) Scheme of Giemsa banding pattern, representing the heterochromatic regions. (B) Fluorescence bands after staining with DAPI. (C) FISH with tandemly repeated Fok elements (59 bp, red). (D) Immunostaining of H4Ac5. Note that the acetylation is stronger at the NOR and weaker at the interstitial heterochromatin than at the euchromatin. The same pattern was obtained with antibodies against H4Ac8 and H4Ac12. (E) Immunostaining of H4Ac16. Chromosome V is used to illustrate the three types of labeling during mitosis: 30% of the chromosomes showed an acetylation pattern identical to that obtained for H4Ac5 (top); 30% showed a uniform acetylation (middle), as described by Belyaev et al. (1997); and 40% revealed more strongly acetylated interstitial heterochromatin (bottom). (F) Immunostaining of H3Ac14. Note the decreased acetylation of Fok element–containing (C) and the increased acetylation of Fok element–free, DAPI-positive (B) interstitial heterochromatic regions in comparison with euchromatin. The same pattern was obtained also for H3Ac9/18.
Figure 2.
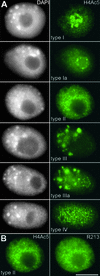
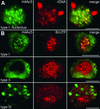
Types of Immunolabeling Pattern of Field Bean Interphase Nuclei Obtained with Antiserum against Histone H4Ac5. (A) Nuclei counterstained with DAPI (left) and after immunolabeling of H4Ac5 (right). Note the different labeling intensities of nucleoli (strong in types I, Ia, and IIIa but absent in types II and III) and the additional “empty” spots (types I, Ia, II, and IV) or “bright” signal spots (types III and IIIa) in chromatin. (B) Type II nucleus with nucleolus free of H4Ac5 (left) and also nearly free of immunosignals after subsequent labeling with antiserum R213 (right), which recognizes H4 regardless of acetylation. The absence of this label indicates depletion of H4 and therefore the absence of complete nucleosomes within nucleoli of these types of nuclei; the same was true for nucleoli of type III nuclei. Bar = 10 μm.
Figure 3.
Histogram of Relative DNA Content of Unsynchronized Field Bean Root Tip Nuclei after DAPI Staining and Flow-Cytometric Analysis. The gates (representing G1, early S, mid-S, late S, and G2 phases) used for sorting are as indicated.
Figure 4.
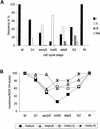
Variation of Histone H4 Acetylation Patterns during the Cell Cycle. (A) Proportion of nuclei of labeling types I, II, III, and IIIa after immunodetection of H4Ac5 in different cell cycle stages. Because types Ia and IV revealed a nearly constant frequency, ranging from 5 to 14% and 5 to 6%, respectively, they therefore were omitted (cf. with Table 1). (B) Relative frequency of nuclei showing acetylation of lysines 5, 8, 12, and 16 of H4 inside nucleoli during the cell cycle.
Figure 5.
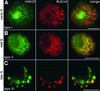
Correlation of Histone Acetylation (H4Ac5) and DNA Replication during S Phase. After 30 min of BrdUrd pulse, the nuclei were isolated, flow-sorted, and double-immunolabeled for H4Ac5 (green, left) and BrdUrd (red, middle). (A) Early S phase. (B) Mid-S phase. (C) Late S phase. Note the large degree of colocalization of both immunosignals in early S (except for the NOR), mid-S, and late S nuclei. The bright spots in (C) represent late-replicating heterochromatin. .
Figure 6.
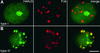
Histone H4 Acetylation of Interstitial Heterochromatin Changes during the Cell Cycle. (A) FISH with Fok elements (red, middle) performed after H4Ac5 immunolabeling (green, left) shows that heterochromatin domains coincide with empty spots representing underacetylation in type I nuclei, that is, during G1 and G2 (cf. with Table 1); the same is true for nuclei of types Ia, II, and IV. (B) In nuclei of type III (and IIIa), which appear during late S and early G2 (cf. with Table 1 and Figure 4A), late-replicating heterochromatin domains colocalize with bright spots of H4Ac5 labeling. .
Figure 7.
Images of a Type III Nucleus of Late S to Early G2 Phase after Immunodetection and FISH. (A) and (B) Immunodetection of H4Ac5 (green, left) followed by FISH with Fok elements (red, right) as observed under (A) epifluorescence and (B) confocal microscopy overlaying 13 optical sections through the nucleus. H4Ac5 immunosignals were captured. (A) Before FISH. (B) After FISH. The major immuno- and FISH signals are identical within both images. The green signal covering the nucleolus in (B) is autofluorescence, which frequently appeared when images were taken after FISH, although it was absent from the same nuclei when checked before FISH, as in (A). .
Figure 8.
Histone Acetylation and Transcriptional Activity of rDNA during Interphase. (A) FISH with rDNA (red, middle) after immunolabeling of H4Ac5 (green, left) from a type I nucleus (only the nucleolus is shown) characteristic for G1 and G2 stages. Perinucleolar knobs containing inactive rDNA and some foci of condensed rDNA inside nucleoli are free of H4Ac5, as shown after merging of both signals. Most of H4Ac5 immunosignals are confined to faint, diffuse rDNA signals. (B) Immunostaining of H4Ac5 (left), BrUTP incorporation (middle), and merging of both signals (right) for type I (G1), II (mid-S), and III (late S) nuclei. The transcriptional activity of rDNA is not correlated with H4 acetylation. Nucleoli are heavily labeled already after 4 min of BrUTP incorporation (red), irrespective of their acetylation status (green). Although BrUTP signals outside nucleoli are much weaker, no transcription signals were detected within heterochromatin domains (neither within empty spots in type II nuclei nor within bright spots in type III nuclei). ;
.
Figure 9.
H3Ac14 Labeling Patterns of Field Bean Interphase Nuclei and Their Correlation with Heterochromatic Domains. (A) Nucleus with bright signal spots for H3Ac14 and labeled nucleolus. This type represents the majority (63 to 74%) of nuclei in all interphase stages. (B) Nucleus with bright signal spots but without intense labeling of the nucleolus. (C) Nucleus without bright signal spots; the nucleolus is somewhat more strongly labeled than the remaining chromatin. (D) Nucleus with neither bright signal spots nor intensely labeled nucleolus. DAPI staining (left) and immunodetection (right). Note the presence of more weakly labeled areas in all nuclei and the correlation of bright signal spots with areas of positive DAPI fluorescence. The frequencies of these types in the course of interphase are given in Table 2. (E) Same type of nucleus as in (B) after immunodetection of H3Ac14 (left), FISH with Fok elements (middle), and merging of both (right). Fok element sites (red) occupy the less acetylated areas and do not colocalize with the bright signal spots for H3Ac14, which represent Fok element–free heterochromatin. .
Figure 10.
Acetylation of Nucleosomal Histones at the NOR, Euchromatic, and Heterochromatic Domains of the Field Bean during the Cell Cycle. (A) Histone H4. A strong cell cycle–dependent histone H4 acetylation occurs at the level of distinct chromatin domains. Heterochromatin contains acetylated H4 (except H4Ac8) during and (shortly) after replication; euchromatin, too, is most strongly acetylated during replication; the NOR contains acetylated H4 during mitosis, as do nucleoli in G1 and G2, but during S phase the histone H4 acetylation within nucleoli is considerably decreased. (B) Histone H3. The intensity of H3 acetylation differs between Fok element–free heterochromatin, Fok element–containing heterochromatin, euchromatin, and the NOR/nucleolus but, unlike H4 acetylation, remains fairly constant throughout the cell cycle.
Similar articles
- Chromatin organization and its relation to replication and histone acetylation during the cell cycle in barley.
Jasencakova Z, Meister A, Schubert I. Jasencakova Z, et al. Chromosoma. 2001 May;110(2):83-92. doi: 10.1007/s004120100132. Chromosoma. 2001. PMID: 11453558 - The acetylation patterns of histones H3 and H4 along Vicia faba chromosomes are different.
Belyaev ND, Houben A, Baranczewski P, Schubert I. Belyaev ND, et al. Chromosome Res. 1998 Jan;6(1):59-63. doi: 10.1023/a:1009222609581. Chromosome Res. 1998. PMID: 9510512 - Histone H4 acetylation in plant heterochromatin is altered during the cell cycle.
Belyaev ND, Houben A, Baranczewski P, Schubert I. Belyaev ND, et al. Chromosoma. 1997 Aug;106(3):193-7. doi: 10.1007/s004120050239. Chromosoma. 1997. PMID: 9233993 - Acetylation of yeast histone H4 lysine 16: a switch for protein interactions in heterochromatin and euchromatin.
Millar CB, Kurdistani SK, Grunstein M. Millar CB, et al. Cold Spring Harb Symp Quant Biol. 2004;69:193-200. doi: 10.1101/sqb.2004.69.193. Cold Spring Harb Symp Quant Biol. 2004. PMID: 16117649 Review. No abstract available. - Linking replication stress with heterochromatin formation.
Nikolov I, Taddei A. Nikolov I, et al. Chromosoma. 2016 Jun;125(3):523-33. doi: 10.1007/s00412-015-0545-6. Epub 2015 Oct 28. Chromosoma. 2016. PMID: 26511280 Free PMC article. Review.
Cited by
- Sex chromatin and nucleolar analyses in Rumex acetosa L.
Lengerova M, Vyskot B. Lengerova M, et al. Protoplasma. 2001;217(4):147-53. doi: 10.1007/BF01283395. Protoplasma. 2001. PMID: 11732306 - Expression of Two Rye CENH3 Variants and Their Loading into Centromeres.
Evtushenko EV, Elisafenko EA, Gatzkaya SS, Schubert V, Houben A, Vershinin AV. Evtushenko EV, et al. Plants (Basel). 2021 Sep 28;10(10):2043. doi: 10.3390/plants10102043. Plants (Basel). 2021. PMID: 34685852 Free PMC article. - NRPD4, a protein related to the RPB4 subunit of RNA polymerase II, is a component of RNA polymerases IV and V and is required for RNA-directed DNA methylation.
He XJ, Hsu YF, Pontes O, Zhu J, Lu J, Bressan RA, Pikaard C, Wang CS, Zhu JK. He XJ, et al. Genes Dev. 2009 Feb 1;23(3):318-30. doi: 10.1101/gad.1765209. Genes Dev. 2009. PMID: 19204117 Free PMC article. - Arabidopsis kinetochore null2 is an upstream component for centromeric histone H3 variant cenH3 deposition at centromeres.
Lermontova I, Kuhlmann M, Friedel S, Rutten T, Heckmann S, Sandmann M, Demidov D, Schubert V, Schubert I. Lermontova I, et al. Plant Cell. 2013 Sep;25(9):3389-404. doi: 10.1105/tpc.113.114736. Epub 2013 Sep 6. Plant Cell. 2013. PMID: 24014547 Free PMC article. - Chromatin Ring Formation at Plant Centromeres.
Schubert V, Ruban A, Houben A. Schubert V, et al. Front Plant Sci. 2016 Feb 15;7:28. doi: 10.3389/fpls.2016.00028. eCollection 2016. Front Plant Sci. 2016. PMID: 26913037 Free PMC article.
References
- Belyaev, N.D., Keohane, A.M., and Turner, B.M. (1996). Differential underacetylation of histones H2A, H3 and H4 on the inactive X chromosome in human female cells. Hum. Genet. 97, 573–578. - PubMed
- Belyaev, N.D., Houben, A., Baranczewski, P., and Schubert, I. (1997). Histone H4 acetylation in plant heterochromatin is altered during the cell cycle. Chromosoma 106, 193–197. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials