Genomic targeting of methylated DNA: influence of methylation on transcription, replication, chromatin structure, and histone acetylation - PubMed (original) (raw)
Genomic targeting of methylated DNA: influence of methylation on transcription, replication, chromatin structure, and histone acetylation
D Schübeler et al. Mol Cell Biol. 2000 Dec.
Abstract
We have developed a strategy to introduce in vitro-methylated DNA into defined chromosomal locations. Using this system, we examined the effects of methylation on transcription, chromatin structure, histone acetylation, and replication timing by targeting methylated and unmethylated constructs to marked genomic sites. At two sites, which support stable expression from an unmethylated enhancer-reporter construct, introduction of an in vitro-methylated but otherwise identical construct results in specific changes in transgene conformation and activity, including loss of the promoter DNase I-hypersensitive site, localized hypoacetylation of histones H3 and H4 within the reporter gene, and a block to transcriptional initiation. Insertion of methylated constructs does not alter the early replication timing of the loci and does not result in de novo methylation of flanking genomic sequences. Methylation at the promoter and gene is stable over time, as is the repression of transcription. Surprisingly, sequences within the enhancer are demethylated, the hypersensitive site forms, and the enhancer is hyperacetylated. Nevertheless, the enhancer is unable to activate the methylated and hypoacetylated reporter. Our findings suggest that CpG methylation represses transcription by interfering with RNA polymerase initiation via a mechanism that involves localized histone deacetylation. This repression is dominant over a remodeled enhancer but neither results in nor requires region-wide changes in DNA replication or chromatin structure.
Figures
FIG. 1
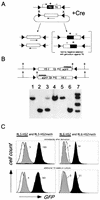
(A) Principle of Cre-RMCE with inverted loxP sites. First, a stable cell line is generated with a construct encoding the positive-negative selectable marker gene HYTK (a fusion of hygromycin B phosphotransferase and herpes simplex virus thymidine kinase) flanked by inverted loxP sites. For the replacement reaction, a construct containing a similar set of loxP sites flanking the cassette to be recombined is transfected together with a Cre recombinase expression plasmid. Recombination between the loxP sites in the two constructs results in exchange of the cassettes and loss of the TK-negative selectable marker. The inverted loxP sites on the same DNA molecule can also recombine, resulting in the inversion of the intervening DNA (10). Ganciclovir is used to select against cells that still express the HYTK gene, allowing isolation of cells that have undergone the targeting reaction. (B) Representative Southern blot analysis of clones derived from RL6 using a restriction enzyme and probe combination that allows determination of the correct integration and orientation. Clones containing the transgene exclusively in one orientation (lanes 1, 2, 4, 5, and 6) were further analyzed, a mixture of both orientations (lane 3), or additional random insertions of the targeting construct (lane 7) were discarded. (C) Expression of the reporter gene, as measured by flow cytometry, is independent of the time in culture and is repressed by in vitro methylation. After targeted insertion into RL5 and RL6, clones containing the unmethylated transgene (RL5-HS2 and RL6-HS2 [black]) or the in vitro-methylated transgene (RL5-HS2meth and RL6-HS2meth [grey]) were analyzed by FACS either immediately after derivation (upper profile) or after an additional 10 weeks in culture (lower profile). The original RL5 and RL6 clones (containing only the HYTK marker) served as a negative control (white). The fluorescence values shown reflect the difference between the median of the transgene containing clone and that of the GFP negative parental clone.
FIG. 2
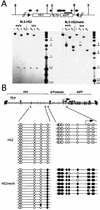
Maintenance of the methylation status. (A) Map of the L1-HS2GFP-1L transgene, including locations of the _Hpa_II (black diamonds) _Xba_I (X), _Bgl_II (B), and loxP sites (black triangles). For Southern blot analyses, genomic DNA from the early and late time points was digested with either _Xba_I or _Bgl_II, in combination with the methylation-sensitive enzyme _Hpa_II, and hybridized with a GFP probe (black bar). The unmethylated clone RL5-HS2 yields a 600-bp fragment with both _Hpa_II-containing digests at both time points, indicating that no de novo methylation of the GFP gene has occurred. The in vitro-methylated clone RL5-HS2meth shows methylation of all _Hpa_II sites in the transgene, with the exception of the three _Hpa_II sites at the 5′ end of the transgene, as indicated by the 2.4-kb fragment obtained with a _Bgl_II/_Hpa_II digest (see the text). (B) Detailed mapping of the methylation status of the enhancer and promoter region. Genomic DNA from the late time point was bisulfite converted, and the sequences of interest were PCR amplified, subcloned, and sequenced (see Materials and Methods). The positions of primers are indicated by open triangles. Open or filled circles correspond to unmethylated or methylated CpGs, respectively.
FIG. 3
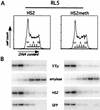
Replication timing of the RL5 insertion site containing the unmethylated (RL5-HS2) and methylated transgene (RL5-HS2meth). (A) Histograms of PI staining intensity (DNA content) of cells for timing analysis are shown. The gates used to sort cells into fractions corresponding approximately to G1, S phase (S1 to S4), and G2 are labeled. (B) PCR-Southern analysis of replication timing of the transgene and control loci. Analysis was performed as described in Materials and Methods, using primers for the early-replicating control loci (endogenous murine β-globin [5′Ey3+4]), a late-replicating control locus (murine amylase [mAmyl1+2]), and the transgene enhancer (huHS21+2) and reporter gene (roGFP1+2). In RL5-HS2 and RL5-HS2meth, the transgene replicates as early as the endogenous murine β-globin locus, indicating that methylation of the transgene does not delay its replication timing.
FIG. 4
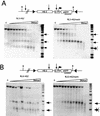
Analysis of enhancer and promoter remodeling. Nuclei were isolated and digested with increasing concentrations of DNase I. Subsequently, genomic DNA was isolated, digested with Bgl II, and hybridized with a probe corresponding to the GFP gene. (A) Analysis of integration site RL5 with the unmethylated (RL5-HS2) and methylated (RL5-HS2meth) transgene. (B) Analysis of integration site RL6. Each hypersensitive site detected is marked with an arrow.
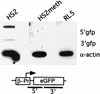
FIG. 5
Nuclear run-on analysis to determine the density of polymerases in the unmethylated and methylated transgenes at the RL5 integration site. Nuclei from the RL5-HS2 clone, containing the unmethylated transgene, the RL5-HS2meth clone, containing the methylated transgene and the control parental clone RL5 without the transgene were isolated. Nuclear run-on assays were performed in the presence of radioactively labeled CTP, and nascent RNA was hybridized to three different DNA probes: α-actin as an endogenous control; 5′GFP, a promoter-proximal fragment containing the 3′ end of the β-globin promoter and the 5′ half of the GFP gene (a PCR product generated with the primer pair roGFP1+2); and 3′GFP, a promoter-distal fragment containing most of the 3′ half of the GFP coding region (generated with the primer pair GFP1+2). The actively expressing clone RL5-HS2 yields a higher signal for the proximal than for the distal probe, indicating that promoter-proximal pausing of polymerases occurs (see the text). In contrast, RL5-HS2meth gives no signal above background for either probes, suggesting a strong reduction of polymerase loading in the methylated state.
FIG. 6
Chromatin immunoprecipitation analysis of histone H3 and H4 acetylation in different regions of the methylated and unmethylated transgene. Antibodies recognizing all acetylated isoforms of H4 (H4) or histone H3 acetylated at lysines 9 and 14 (H3) were used for immunoprecipitation. PCRs were performed on the input and antibody-bound chromatin fractions in the presence of a radiolabeled nucleotide under conditions of linear amplifications, as we have shown previously (see reference and Materials and Methods). One primer pair amplifies a sequence from the transgene and the other amplifies a sequence from the endogenous mouse amylase 2.1y gene. The PCR products from the input (I) and antibody-bound DNA (H3 and H4) were electrophoresed on a nondenaturing acrylamide gel; a representative gel is shown. (B) Quantification of duplex PCR products from three independent immunoprecipitation experiments. The transgene/amylase ratio from each bound fraction was standardized by dividing by the transgene/amylase ratio from the input material to determine the relative enrichment of transgenic sequences during the immunoprecipitation. The mean value and standard error of the mean for the enrichment are plotted (see the text). The x axis is drawn at 1, which reflects no enrichment.
Similar articles
- Methylation-mediated proviral silencing is associated with MeCP2 recruitment and localized histone H3 deacetylation.
Lorincz MC, Schübeler D, Groudine M. Lorincz MC, et al. Mol Cell Biol. 2001 Dec;21(23):7913-22. doi: 10.1128/MCB.21.23.7913-7922.2001. Mol Cell Biol. 2001. PMID: 11689684 Free PMC article. - Silencing of transgene transcription precedes methylation of promoter DNA and histone H3 lysine 9.
Mutskov V, Felsenfeld G. Mutskov V, et al. EMBO J. 2004 Jan 14;23(1):138-49. doi: 10.1038/sj.emboj.7600013. Epub 2003 Dec 11. EMBO J. 2004. PMID: 14685282 Free PMC article. - The barrier function of an insulator couples high histone acetylation levels with specific protection of promoter DNA from methylation.
Mutskov VJ, Farrell CM, Wade PA, Wolffe AP, Felsenfeld G. Mutskov VJ, et al. Genes Dev. 2002 Jun 15;16(12):1540-54. doi: 10.1101/gad.988502. Genes Dev. 2002. PMID: 12080092 Free PMC article. - Enhancer RNA: biogenesis, function, and regulation.
Ye R, Cao C, Xue Y. Ye R, et al. Essays Biochem. 2020 Dec 7;64(6):883-894. doi: 10.1042/EBC20200014. Essays Biochem. 2020. PMID: 33034351 Review. - A Comprehensive Toolbox to Analyze Enhancer-Promoter Functions.
Giaimo BD, Friedrich T, Borggrefe T. Giaimo BD, et al. Methods Mol Biol. 2021;2351:3-22. doi: 10.1007/978-1-0716-1597-3_1. Methods Mol Biol. 2021. PMID: 34382181 Review.
Cited by
- Application of microdroplet PCR for large-scale targeted bisulfite sequencing.
Komori HK, LaMere SA, Torkamani A, Hart GT, Kotsopoulos S, Warner J, Samuels ML, Olson J, Head SR, Ordoukhanian P, Lee PL, Link DR, Salomon DR. Komori HK, et al. Genome Res. 2011 Oct;21(10):1738-45. doi: 10.1101/gr.116863.110. Epub 2011 Jul 14. Genome Res. 2011. PMID: 21757609 Free PMC article. - Aberrant epigenetic silencing is triggered by a transient reduction in gene expression.
Oyer JA, Chu A, Brar S, Turker MS. Oyer JA, et al. PLoS One. 2009;4(3):e4832. doi: 10.1371/journal.pone.0004832. Epub 2009 Mar 12. PLoS One. 2009. PMID: 19279688 Free PMC article. - Dynamic heterogeneity and DNA methylation in embryonic stem cells.
Singer ZS, Yong J, Tischler J, Hackett JA, Altinok A, Surani MA, Cai L, Elowitz MB. Singer ZS, et al. Mol Cell. 2014 Jul 17;55(2):319-31. doi: 10.1016/j.molcel.2014.06.029. Mol Cell. 2014. PMID: 25038413 Free PMC article. - Impact of light on Hypocrea jecorina and the multiple cellular roles of ENVOY in this process.
Schuster A, Kubicek CP, Friedl MA, Druzhinina IS, Schmoll M. Schuster A, et al. BMC Genomics. 2007 Dec 4;8:449. doi: 10.1186/1471-2164-8-449. BMC Genomics. 2007. PMID: 18053205 Free PMC article. - Unraveling the neurotoxicity of titanium dioxide nanoparticles: focusing on molecular mechanisms.
Song B, Zhang Y, Liu J, Feng X, Zhou T, Shao L. Song B, et al. Beilstein J Nanotechnol. 2016 Apr 29;7:645-54. doi: 10.3762/bjnano.7.57. eCollection 2016. Beilstein J Nanotechnol. 2016. PMID: 27335754 Free PMC article. Review.
References
- Bird A P, Wolffe A P. Methylation-induced repression—belts, braces, and chromatin. Cell. 1999;99:451–454. - PubMed
- Brandeis M, Ariel M, Cedar H. Dynamics of DNA methylation during development. Bioessays. 1993;15:709–713. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM 19767/01/GM/NIGMS NIH HHS/United States
- HL554350/HL/NHLBI NIH HHS/United States
- R01 DK056845/DK/NIDDK NIH HHS/United States
- R37 DK044746/DK/NIDDK NIH HHS/United States
- F32 GM019767/GM/NIGMS NIH HHS/United States
- HL38655/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources