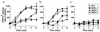Ionophore-resistant mutants of Toxoplasma gondii reveal host cell permeabilization as an early event in egress - PubMed (original) (raw)
Ionophore-resistant mutants of Toxoplasma gondii reveal host cell permeabilization as an early event in egress
M W Black et al. Mol Cell Biol. 2000 Dec.
Abstract
Toxoplasma gondii is an obligate intracellular pathogen within the phylum Apicomplexa. Invasion and egress by this protozoan parasite are rapid events that are dependent upon parasite motility and appear to be directed by fluctuations in intracellular [Ca(2+)]. Treatment of infected host cells with the calcium ionophore A23187 causes the parasites to undergo rapid egress in a process termed ionophore-induced egress (IIE). In contrast, when extracellular parasites are exposed to this ionophore, they quickly lose infectivity (termed ionophore-induced death [IID]). From among several Iie(-) mutants described here, two were identified that differ in several attributes, most notably in their resistance to IID. The association between the Iie(-) and Iid(-) phenotypes is supported by the observation that two-thirds of mutants selected as Iid(-) are also Iie(-). Characterization of three distinct classes of IIE and IID mutants revealed that the Iie(-) phenotype is due to a defect in a parasite-dependent activity that normally causes infected host cells to be permeabilized just prior to egress. Iie(-) parasites underwent rapid egress when infected cells were artificially permeabilized by a mild saponin treatment, confirming that this step is deficient in the Iie(-) mutants. A model is proposed that includes host cell permeabilization as a critical part of the signaling pathway leading to parasite egress. The fact that Iie(-) mutants are also defective in early stages of the lytic cycle indicates some commonality between these normal processes and IIE.
Figures
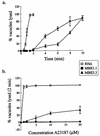
FIG. 1
The IIE phenotype of wild-type (RHΔ) and two Iie− mutants (MBE1.1 and MBE3.3). (a) A time course of egress using 1 μM A23187 in HBSSc. (b) IIE as a function of A23187 concentration. Egress was measured after 2 min of exposure to the ionophore. Monolayers for both panels a and b were scored for the percentage of lysed vacuoles per field.
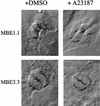
FIG. 2
A23187 induction of parasite elongation in intracellular Iie− mutants. Infected cells were treated with DMSO (left) or 1 μM A23187 (right) in HBSSc and were fixed after 2 min at 37°C. The morphological phenotype is observed as an extension of the apical end (pointing out from the center of the vacuoles). Intracellular MBE1.1 parasites demonstrate this phenotype, while those of the MBE3.3 strain remain rounded.
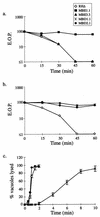
FIG. 3
Relationship between the IIE and IID phenotypes. (a) MBE1.1, but not MBE3.3, is resistant to IID. Extracellular parasites were exposed to A23187 and assayed for the formation of plaques. EOP was defined as the percentage of the PFU arising from parasites incubated with A23187 versus the DMSO-treated control. (b) Iid− phenotypes of MBD1.1 and MBD2.1, two independent mutants isolated in the Iid− selection. Assays were performed as in panel a. (c) IIE in the Iid− selected mutants. MBD1.1 is Iie−, while MBD2.1 is Iie+. The IIE assay was performed as described for Fig. 1a.
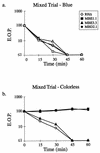
FIG. 4
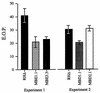
IID is not due to a freely diffusible lytic activity. A strain of RHΔ that was transformed with a SAG1-driven β-galactosidase reporter (24; RHΔ-βgal) was used as the Iid+ strain and mixed with RHΔ, MBE1.1, MBE3.3, or MBD2.1 in a 1:1 ratio. The extracellular parasites were incubated in 1 μM A23187 in HBSSc at 37°C for periods of 0 to 60 min, diluted, and plated on HFF monolayers for the development of plaques. After 5 days, the monolayers were fixed and stained with 5-bromo-4-chloro-3-indolyl-β-
d
-galactopyranoside (X-Gal) to distinguish the plaques from each strain. After counting the blue plaques, the monolayer was counterstained with crystal violet to score all the remaining “colorless” plaques. (a) Control showing the expected drop in EOP of the Iid+ RHΔ-βgal strain in the four coincubation assays from panel b (scoring for blue plaques). (b) The effect of coincubation with RHΔ-βgal on the IID phenotype of the four strains.
FIG. 5
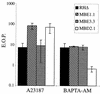
The effect on EOP of pretreating extracellular parasites with either A23187 or BAPTA-AM. Extracellular parasites were pretreated with either 1 μM A23187 or 50 μM BAPTA-AM in HBSSc for 30 min at 37°C and were plated to determine the EOP expressed as the percent relative to treatment with DMSO alone. The data are pooled from three independent trials. The two Iid+ strains (RHΔ and MBE3.3) showed the expected drop in EOP after exposure to A23187. Upon treatment with BAPTA-AM, RHΔ, MBE1.1, and MBE3.3 showed about a 90% drop in EOP while MBD2.1 was hypersensitive to this treatment, with a drop of over 99%.
FIG. 6
EOP of Iie− and Iid− mutants. Parasites were allowed to infect HFF monolayers for 24 h, after which extracellular parasites were removed and the plaques were allowed to develop for 4 more days. Mutants defective in IIE also showed a reduction in the efficiency of plaquing.
FIG. 7
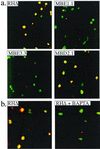
Ionophore-induced membrane permeabilization by intracellular Iie+ parasites. Infected cells were pretreated with cytochalasin D to block parasite motility, and A23187 was added before fixing the monolayer. Immunofluorescence was used to examine the membrane integrity of host cells infected with Iie+ and Iie− strains. A rabbit anti-SAG1 antibody was used before permeabilizing the monolayer with detergent so as to stain all the parasites that are extracellular or within permeabilized host cells. A mouse anti-SAG1 MAb was used in the presence of Triton X-100 to permeabilize all membranes and allow all the parasites to be stained regardless of their location or the state of the host cell. The two antibodies were detected with different fluorescent dyes, rabbit antibodies with Texas red and mouse antibodies with FITC, such that all parasites stained with FITC but only those that were within permeabilized host cells also stained with Texas red and thus appear yellow. (a) The effect of A23187 on the membrane integrity of host cells infected with Iie+ (RHΔ and MBD2.1) and Iie− (MBE1.1 and MBE3.3) strains. Cells infected with RHΔ and MBD2.1 are permeabilized despite the absence of parasite motility. This permeabilization is not observed in cells infected with MBE1.1 and MBE3.3. (b) The A23187-induced permeabilization by the Iie+ RHΔ strain is inhibited by pretreating the infected monolayer with BAPTA-AM.
FIG. 8
Quantitation of egress-independent host lysis using a 51Cr release assay. HFF monolayers were infected with either RHΔ, MBE1.1, MBE3.3, or MBD2.1 and were loaded with 51Cr. Cells were pretreated with either DMSO (a), cytochalasin D (b), or BAPTA-AM (c). A23187 was then added to all cultures, and the counts per minute (CPM) obtained from 51Cr release was measured and normalized to 104 parasitophorous vacuoles.
FIG. 9
Saponin induces egress in a Ca2+-dependent manner. Intracellular parasites were induced to exit by treatment with either 0.005% saponin (a) or 1 μM A23187 (b) at 4°C for 20 min. After an additional 2 min of incubation at 37°C, the cells were fixed and stained. The influence of extraparasitic [Ca2+] on each treatment was examined by using either HBSSc (1 mM Ca2+) or HBSSnc (5 mM EGTA and no added Ca2+).
Similar articles
- A genetic screen to isolate Toxoplasma gondii host-cell egress mutants.
Coleman BI, Gubbels MJ. Coleman BI, et al. J Vis Exp. 2012 Feb 8;(60):3807. doi: 10.3791/3807. J Vis Exp. 2012. PMID: 22349295 Free PMC article. - Toxoplasma gondii: induction of egress by the potassium ionophore nigericin.
Fruth IA, Arrizabalaga G. Fruth IA, et al. Int J Parasitol. 2007 Dec;37(14):1559-67. doi: 10.1016/j.ijpara.2007.05.010. Epub 2007 Jun 9. Int J Parasitol. 2007. PMID: 17618633 Free PMC article. - Toxoplasma gondii: dithiol-induced Ca2+ flux causes egress of parasites from the parasitophorous vacuole.
Stommel EW, Ely KH, Schwartzman JD, Kasper LH. Stommel EW, et al. Exp Parasitol. 1997 Oct;87(2):88-97. doi: 10.1006/expr.1997.4187. Exp Parasitol. 1997. PMID: 9326884 - Calcium signaling and the lytic cycle of the Apicomplexan parasite Toxoplasma gondii.
Hortua Triana MA, Márquez-Nogueras KM, Vella SA, Moreno SNJ. Hortua Triana MA, et al. Biochim Biophys Acta Mol Cell Res. 2018 Nov;1865(11 Pt B):1846-1856. doi: 10.1016/j.bbamcr.2018.08.004. Epub 2018 Aug 10. Biochim Biophys Acta Mol Cell Res. 2018. PMID: 30992126 Free PMC article. Review. - Role of calcium during Toxoplasma gondii invasion and egress.
Arrizabalaga G, Boothroyd JC. Arrizabalaga G, et al. Int J Parasitol. 2004 Mar 9;34(3):361-8. doi: 10.1016/j.ijpara.2003.11.017. Int J Parasitol. 2004. PMID: 15003496 Review.
Cited by
- A genetic screen to isolate Toxoplasma gondii host-cell egress mutants.
Coleman BI, Gubbels MJ. Coleman BI, et al. J Vis Exp. 2012 Feb 8;(60):3807. doi: 10.3791/3807. J Vis Exp. 2012. PMID: 22349295 Free PMC article. - Egress Regulatory Factors: How Toxoplasma Exits from Infected Cells?
Diao Y, Yao Y, El-Ashram S, Bian M. Diao Y, et al. Pathogens. 2023 May 4;12(5):679. doi: 10.3390/pathogens12050679. Pathogens. 2023. PMID: 37242349 Free PMC article. Review. - Externally triggered egress is the major fate of Toxoplasma gondii during acute infection.
Tomita T, Yamada T, Weiss LM, Orlofsky A. Tomita T, et al. J Immunol. 2009 Nov 15;183(10):6667-80. doi: 10.4049/jimmunol.0900516. Epub 2009 Oct 21. J Immunol. 2009. PMID: 19846885 Free PMC article. - The toxoplasma Acto-MyoA motor complex is important but not essential for gliding motility and host cell invasion.
Egarter S, Andenmatten N, Jackson AJ, Whitelaw JA, Pall G, Black JA, Ferguson DJ, Tardieux I, Mogilner A, Meissner M. Egarter S, et al. PLoS One. 2014 Mar 14;9(3):e91819. doi: 10.1371/journal.pone.0091819. eCollection 2014. PLoS One. 2014. PMID: 24632839 Free PMC article. - 4-Bromophenacyl bromide specifically inhibits rhoptry secretion during Toxoplasma invasion.
Ravindran S, Lodoen MB, Verhelst SH, Bogyo M, Boothroyd JC. Ravindran S, et al. PLoS One. 2009 Dec 2;4(12):e8143. doi: 10.1371/journal.pone.0008143. PLoS One. 2009. PMID: 19956582 Free PMC article.
References
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K, editors. Current protocols in molecular biology. 1. J. Boston, Mass: Wiley and Sons, Inc.; 1995.
- Burg J L, Perelman D, Kasper L H, Ware P L, Boothroyd J C. Molecular analysis of the gene encoding the major surface antigen of Toxoplasma gondii. J Immunol. 1988;141:3584–3591. - PubMed
- Carruthers V B, Sibley L D. Mobilization of intracellular calcium stimulates microneme discharge in Toxoplasma gondii. Mol Microbiol. 1999;31:421–428. - PubMed
- Carruthers V B, Sibley L D. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. - PubMed
- Chiappino M L, Nichols B A, O'Connor G R. Scanning electron microscopy of Toxoplasma gondii: parasite torsion and host-cell responses during invasion. J Protozool. 1984;31:288–292. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI21423/AI/NIAID NIH HHS/United States
- T32 AI007328/AI/NIAID NIH HHS/United States
- R01 AI021423/AI/NIAID NIH HHS/United States
- R37 AI021423/AI/NIAID NIH HHS/United States
- AI07328/AI/NIAID NIH HHS/United States
- AI45057/AI/NIAID NIH HHS/United States
- R01 AI045057/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous