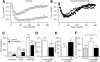Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca(2+) current I(CRAC) - PubMed (original) (raw)
Respiring mitochondria determine the pattern of activation and inactivation of the store-operated Ca(2+) current I(CRAC)
J A Gilabert et al. EMBO J. 2000.
Abstract
In eukaryotic cells, hormones and neurotransmitters that engage the phosphoinositide pathway evoke a biphasic increase in intracellular free Ca(2+) concentration: an initial transient release of Ca(2+) from intracellular stores is followed by a sustained phase of Ca(2+) influx. This influx is generally store dependent. Most attention has focused on the link between the endoplasmic reticulum and store-operated Ca(2+) channels in the plasma membrane. Here, we describe that respiring mitochondria are also essential for the activation of macroscopic store-operated Ca(2+) currents under physiological conditions of weak intracellular Ca(2+) buffering. We further show that Ca(2+)-dependent slow inactivation of Ca(2+) influx, a widespread but poorly understood phenomenon, is regulated by mitochondrial buffering of cytosolic Ca(2+). Thus, by enabling macroscopic store-operated Ca(2+) current to activate, and then by controlling its extent and duration, mitochondria play a crucial role in all stages of store-operated Ca(2+) influx. Store-operated Ca(2+) entry reflects a dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane.
Figures
Fig. 1. ICRAC is smaller using hyperpolarizing steps than ramps in weak intracellular Ca2+ buffer. (A) Time-course of ICRAC, followed using voltage ramps, in strong and weak buffer (filled and open circles, respectively). Inset shows I–V relationships taken at 100 s. The current was measured at –80 mV from the ramps, and normalized for cell capacitance. (B) Time-course of ICRAC in strong and weak buffer following voltage steps to –80 mV (inset). The current was measured between 0.8 and 0.9 ms after the step (see Materials and methods). (C) A plot of ICRAC against activation time-constant in strong buffer using ramps or steps. (D) A similar plot to (C) but for low buffer. Note that inclusion of 2 mM Mg-ATP reduces the size of the current.
Fig. 2. Respiring mitochondria increase the size of ICRAC in weak but not strong buffer. (A) Time-course of ICRAC in weak buffer in the absence (control) and presence of the mitochondrial cocktail. (B) ICRAC is significantly bigger in the presence of cocktail compared with control. (C) Pre-treatment with antimycin A and oligomycin prevents the enhancing effect of cocktail. The experiments were carried out on cells from the same preparations using the same solutions. Cells were incubated for at least 20 min in 5 µg/ml antimycin A and 0.5 µg/ml oligomycin. (D) I–V relationship for a cell dialysed with cocktail when ICRAC had peaked. (E) A plot of current amplitude against % of responding cells in weak and strong buffer in the absence and presence of cocktail. (F) Extent of rapid inactivation in the absence (control) and presence of cocktail. For all panels, control refers to InsP3 + thapsigargin + 0.1 mM EGTA + ATP.
Fig. 3. Inhibition of Ca2+ uptake into respiring mitochondria reduces the extent of ICRAC and accelerates slow inactivation. (A) Time-course of ICRAC in the presence of the mitochondrial cocktail for two cells showing the range of slow inactivation. One showed only modest inactivation (circles), whereas the other inactivated substantially (squares). (B) Time-course of ICRAC in two cells dialysed with 100 µM ruthenium red together with cocktail, showing substantial inactivation. We used this concentration in order to achieve high intracellular levels shortly after breaking into the cells. (C–F) Compare various properties of ICRAC for cocktail (open bars) with cocktail + ruthenium red (filled bars). Apart from τ-activation, ruthenium red significantly reduced all parameters compared with cocktail alone. Time to inactivate was measured from the time at which ICRAC had peaked to the time where inactivation had reached a steady state. The difference between peak current and steady-state current was taken as the inactivation % after normalization to peak amplitude.
Fig. 4. ICRAC activates to InsP3 alone in weak buffer provided mitochondria are active. (A) Time-course of ICRAC for a cell dialysed with InsP3 + 0.1 mM EGTA + ATP (control) and then for one supplemented with cocktail. No thapsigargin was present. (B) I–V relationship from voltage ramps is shown (taken when after 60 s). (C) Bar chart depicting the enhancing effect of cocktail, and that the mitochondrial inhibitors antimycin A (+ oligomycin) and ruthenium red prevent the effects of cocktail. Control + cAMP or GTP did not mimic the effects of cocktail. Again, thapsigargin was not present. (D) Plot of amplitude of ICRAC versus % responding cells for control and cocktail-treated cells (no thapsigargin present). Only the amplitudes of responding cells were analysed. (E) τ-activation versus amplitude of ICRAC is plotted for cocktail-treated cells in weak buffer (no thapsigargin) and strong buffer (InsP3 + thapsigargin + 10 mM EGTA + ATP). Note that in weak buffer without thapsigargin, ICRAC was still sub-maximal in spite of cocktail. This reflects some SERCA-dependent refilling, because the current was larger in weak buffer when thapsigargin was included.
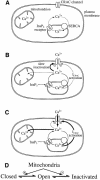
Fig. 5. Cartoon summary of mitochondrial role in ICRAC in weak (physiological) intracellular Ca2+ buffer. (A) Shows the resting state, where ICRAC is not functioning. Stores are full and any Ca2+ that leaks from the stores is re-sequestrated by the SERCA pumps. (B) Following an increase in the levels of the second messenger InsP3 in the absence of active mitochondrial Ca2+ uptake, Ca2+ is released from the stores. However, the SERCA pumps are able to re-sequestrate sufficient Ca2+ to prevent the threshold for macroscopic activation of ICRAC from being reached. Only a very small fraction of CRAC channels are activated (undetectable in whole-cell mode). Furthermore, the rise in cytosolic Ca2+ results in strong inactivation of ICRAC through Ca2+-dependent slow inactivation. (C) In the presence of respiring mitochondria, InsP3 activates macroscopic ICRAC. Ca2+ released from the stores by InsP3 is taken up by mitochondria through a ruthenium red-sensitive uniporter. This reduces the amount of Ca2+ available to the SERCA pumps and in the vicinity of open InsP3 receptors, such that the stores are depleted sufficiently for macroscopic ICRAC to activate (less refilling by SERCA pumps and less inactivation of InsP3 receptors). ICRAC now activates. Some refilling does occur because inclusion of thapsigargin enhances the size of the current. Furthermore, mitochondrial Ca2+ buffering reduces the rate and extent of Ca2+-dependent slow inactivation, thereby increasing the size and duration of the current. (D) A simplified gating scheme for CRAC channels summarizing the role of mitochondrial Ca2+ buffering. Mitochondria facilitate opening (Closed to Open transition) whilst simultaneously reducing inactivation (Open to Inactivated transition). In this way, mitochondria have a much larger impact on ICRAC than through either transition alone.
Similar articles
- Store-operated Ca2+ entry: dynamic interplay between endoplasmic reticulum, mitochondria and plasma membrane.
Parekh AB. Parekh AB. J Physiol. 2003 Mar 1;547(Pt 2):333-48. doi: 10.1113/jphysiol.2002.034140. Epub 2003 Feb 7. J Physiol. 2003. PMID: 12576497 Free PMC article. - Energized mitochondria increase the dynamic range over which inositol 1,4,5-trisphosphate activates store-operated calcium influx.
Gilabert JA, Bakowski D, Parekh AB. Gilabert JA, et al. EMBO J. 2001 Jun 1;20(11):2672-9. doi: 10.1093/emboj/20.11.2672. EMBO J. 2001. PMID: 11387202 Free PMC article. - Store-operated calcium channels.
Parekh AB, Putney JW Jr. Parekh AB, et al. Physiol Rev. 2005 Apr;85(2):757-810. doi: 10.1152/physrev.00057.2003. Physiol Rev. 2005. PMID: 15788710 Review. - Activation of store-operated Ca(2+) channels in trabecular meshwork cells.
Abad E, Lorente G, Gavara N, Morales M, Gual A, Gasull X. Abad E, et al. Invest Ophthalmol Vis Sci. 2008 Feb;49(2):677-86. doi: 10.1167/iovs.07-1080. Invest Ophthalmol Vis Sci. 2008. PMID: 18235014 - Dissecting ICRAC, a store-operated calcium current.
Hogan PG, Rao A. Hogan PG, et al. Trends Biochem Sci. 2007 May;32(5):235-45. doi: 10.1016/j.tibs.2007.03.009. Epub 2007 Apr 16. Trends Biochem Sci. 2007. PMID: 17434311 Review.
Cited by
- The STIM1-ORAI1 microdomain.
Hogan PG. Hogan PG. Cell Calcium. 2015 Oct;58(4):357-67. doi: 10.1016/j.ceca.2015.07.001. Epub 2015 Jul 17. Cell Calcium. 2015. PMID: 26215475 Free PMC article. Review. - Cell proliferation depends on mitochondrial Ca2+ uptake: inhibition by salicylate.
Núñez L, Valero RA, Senovilla L, Sanz-Blasco S, García-Sancho J, Villalobos C. Núñez L, et al. J Physiol. 2006 Feb 15;571(Pt 1):57-73. doi: 10.1113/jphysiol.2005.100586. Epub 2005 Dec 8. J Physiol. 2006. PMID: 16339178 Free PMC article. - Store-operated Ca2+ entry depends on mitochondrial Ca2+ uptake.
Glitsch MD, Bakowski D, Parekh AB. Glitsch MD, et al. EMBO J. 2002 Dec 16;21(24):6744-54. doi: 10.1093/emboj/cdf675. EMBO J. 2002. PMID: 12485995 Free PMC article. - Store-Operated Ca2+ Entry in Tumor Progression: From Molecular Mechanisms to Clinical Implications.
Chen YF, Lin PC, Yeh YM, Chen LH, Shen MR. Chen YF, et al. Cancers (Basel). 2019 Jun 27;11(7):899. doi: 10.3390/cancers11070899. Cancers (Basel). 2019. PMID: 31252656 Free PMC article. Review. - Calcium microdomains at the immunological synapse: how ORAI channels, mitochondria and calcium pumps generate local calcium signals for efficient T-cell activation.
Quintana A, Pasche M, Junker C, Al-Ansary D, Rieger H, Kummerow C, Nuñez L, Villalobos C, Meraner P, Becherer U, Rettig J, Niemeyer BA, Hoth M. Quintana A, et al. EMBO J. 2011 Aug 16;30(19):3895-912. doi: 10.1038/emboj.2011.289. EMBO J. 2011. PMID: 21847095 Free PMC article.
References
- Berridge M.J. (1993) Inositol trisphosphate and calcium signalling. Nature, 361, 315–325. - PubMed
- Broad L.M., Armstrong,D.L. and Putney,J.W. (1999) Role of the inositol 1,4,5-trisphosphate receptor in Ca2+ feedback inhibition of calcium release-activated calcium current (ICRAC). J. Biol. Chem., 274, 32881–32888. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous