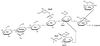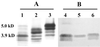Antigenic diversity of Haemophilus somnus lipooligosaccharide: phase-variable accessibility of the phosphorylcholine epitope - PubMed (original) (raw)
Antigenic diversity of Haemophilus somnus lipooligosaccharide: phase-variable accessibility of the phosphorylcholine epitope
M D Howard et al. J Clin Microbiol. 2000 Dec.
Abstract
The lipooligosaccharide (LOS) of Haemophilus somnus undergoes antigenic phase variation, which may facilitate evasion from the bovine host immune response and/or colonization and dissemination. However, LOS antigenic diversity in H. somnus has not been adequately investigated. In this study, monoclonal antibodies (MAbs) specific to various LOS epitopes were used to investigate antigenic variation and stability in LOS from H. somnus strains and phase variants. Clinical isolates of H. somnus exhibited intrastrain, as well as interstrain, antigenic heterogeneity in LOS when probed with MAbs to outer core oligosaccharide epitopes in an enzyme-linked immunosorbent assay (ELISA). However, epitopes reactive with MAbs directed predominately to the inner core heptose region were highly conserved. At least one epitope, which was expressed in few strains, was identified. One LOS component affected by phase variation was identified as phosphorylcholine (PCho), which is linked to the primary glucose residue. Inhibition ELISA, immunoblotting, and electrospray-mass spectrometry were used to confirm that MAb 5F5.9 recognized PCho. LOS reactivity with MAb 5F5.9 was associated with loss of most of the outer core oligosaccharide, indicating that reactivity with PCho was affected by phase variation of the glucose residues in this region. Our results indicate that outer core epitopes of H. somnus LOS exhibit a high degree of random, phase-variable antigenic heterogeneity and that such heterogeneity must be considered in the design of vaccines and diagnostic tests.
Figures
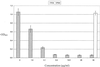
FIG. 1
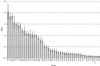
Reactivity of MAb 5F5.9 with strains of H. somnus by ELISA. Error bars, ±2 standard deviations from the mean of the results from four replicate experiments. E. coli J5 is the negative control.
FIG. 2
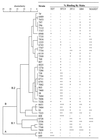
Dendrogram obtained from cluster analysis of all H. somnus strains (four replicate experiments) and the relative percent binding by ELISA with each corresponding MAb. Semiquantitative binding was determined as previously described (29). Symbols: −, +, ++, and +++ represent <15%, ≥15 to 50%, >50 to 90%, and >90% of maximum optical density, respectively.
FIG. 3
Modified Haworth projection of the predominant structure of H. somnus strain 738 LOS (9). All outer core hexoses are beta-D oriented and both inner core
l
-glycero-
d
-manno heptoses are
l
-α-
d
oriented. Abbreviations: Gal, galactose; GlcNAc, _N_-acetyl-glucosamine; Glc, glucose; Hep, heptose; Kdo, 3-deoxy-
d
-_manno_-2-octulosonic acid.
FIG. 4
Silver-stained polyacrylamide gel (A) and immunoblot with MAb 5D7 (B) of H. somnus LOS extracts. Lanes 1 and 4, strain 649; 2 and 5, strain 6948; 3 and 6, strain 7226. Molecular sizes are indicated on the left (23).
FIG. 5
Inhibition ELISA of MAb 5F5.9 by PCho (hatched bars) or PEtn (open bar). H. somnus strain 93 LOS was used as the antigen. Error bars, 2 standard deviations above and below the mean data point from five replicate experiments.
FIG. 6
Silver-stained polyacrylamide gel (A) and immunoblot (B) analyses of LOS extracts from MAb 5F5.9-positive and -negative clonal isolates of H. somnus strain 738. Lanes: 1 and 3, 738-P LOS; 2 and 4, 738 LOS; 5, immunoblot repeated on membrane cut from lane 4 using anti-738 LOS rabbit serum as the primary antibody. Molecular sizes are indicated on the left (23).
Similar articles
- Phase variation and conservation of lipooligosaccharide epitopes in Haemophilus somnus.
Inzana TJ, Hensley J, McQuiston J, Lesse AJ, Campagnari AA, Boyle SM, Apicella MA. Inzana TJ, et al. Infect Immun. 1997 Nov;65(11):4675-81. doi: 10.1128/iai.65.11.4675-4681.1997. Infect Immun. 1997. PMID: 9353049 Free PMC article. - Incorporation of N-acetylneuraminic acid into Haemophilus somnus lipooligosaccharide (LOS): enhancement of resistance to serum and reduction of LOS antibody binding.
Inzana TJ, Glindemann G, Cox AD, Wakarchuk W, Howard MD. Inzana TJ, et al. Infect Immun. 2002 Sep;70(9):4870-9. doi: 10.1128/IAI.70.9.4870-4879.2002. Infect Immun. 2002. PMID: 12183531 Free PMC article. - Phenotypic phase variation in Haemophilus somnus lipooligosaccharide during bovine pneumonia and after in vitro passage.
Inzana TJ, Gogolewski RP, Corbeil LB. Inzana TJ, et al. Infect Immun. 1992 Jul;60(7):2943-51. doi: 10.1128/iai.60.7.2943-2951.1992. Infect Immun. 1992. PMID: 1612761 Free PMC article. - Structurally defined epitopes of Haemophilus ducreyi lipooligosaccharides recognized by monoclonal antibodies.
Ahmed HJ, Frisk A, Månsson JE, Schweda EK, Lagergård T. Ahmed HJ, et al. Infect Immun. 1997 Aug;65(8):3151-8. doi: 10.1128/iai.65.8.3151-3158.1997. Infect Immun. 1997. PMID: 9234768 Free PMC article. - The Many Facets of Lipooligosaccharide as a Virulence Factor for Histophilus somni.
Inzana TJ. Inzana TJ. Curr Top Microbiol Immunol. 2016;396:131-48. doi: 10.1007/82_2015_5020. Curr Top Microbiol Immunol. 2016. PMID: 26814887 Review.
Cited by
- Contribution of Hfq to gene regulation and virulence in Histophilus somni.
Cao D, Subhadra B, Lee Y-J, Thoresen M, Cornejo S, Olivier A, Woolums A, Inzana TJ. Cao D, et al. Infect Immun. 2024 Mar 12;92(3):e0003824. doi: 10.1128/iai.00038-24. Epub 2024 Feb 23. Infect Immun. 2024. PMID: 38391206 Free PMC article. - Exogenous carbon monoxide suppresses Escherichia coli vitality and improves survival in an Escherichia coli-induced murine sepsis model.
Shen WC, Wang X, Qin WT, Qiu XF, Sun BW. Shen WC, et al. Acta Pharmacol Sin. 2014 Dec;35(12):1566-76. doi: 10.1038/aps.2014.99. Epub 2014 Nov 17. Acta Pharmacol Sin. 2014. PMID: 25399652 Free PMC article. - Histophilus somni causes extracellular trap formation by bovine neutrophils and macrophages.
Hellenbrand KM, Forsythe KM, Rivera-Rivas JJ, Czuprynski CJ, Aulik NA. Hellenbrand KM, et al. Microb Pathog. 2013 Jan;54:67-75. doi: 10.1016/j.micpath.2012.09.007. Epub 2012 Sep 27. Microb Pathog. 2013. PMID: 23022668 Free PMC article. - The role of lipooligosaccharide phosphorylcholine in colonization and pathogenesis of Histophilus somni in cattle.
Elswaifi SF, Scarratt WK, Inzana TJ. Elswaifi SF, et al. Vet Res. 2012 Jun 7;43(1):49. doi: 10.1186/1297-9716-43-49. Vet Res. 2012. PMID: 22676226 Free PMC article. - Identification, structure, and characterization of an exopolysaccharide produced by Histophilus somni during biofilm formation.
Sandal I, Inzana TJ, Molinaro A, De Castro C, Shao JQ, Apicella MA, Cox AD, St Michael F, Berg G. Sandal I, et al. BMC Microbiol. 2011 Aug 19;11:186. doi: 10.1186/1471-2180-11-186. BMC Microbiol. 2011. PMID: 21854629 Free PMC article.
References
- Ahmed H J, Borrelli S, Jonasson J, Eriksson L, Hanson S, Hojer B, Sunkuntu M, Musaba E, Roggen E L, Lagergård T, et al. Monoclonal antibodies against Haemophilus ducreyi lipooligosaccharide and their diagnostic usefulness. Eur J Clin Microbiol Infect Dis. 1995;14:892–898. - PubMed
- Briles D E, Forman C, Hudak S, Claflin J L. The effects of idiotype on the ability of IgG1 anti-phosphorylcholine antibodies to protect mice from fatal infection with Streptococcus pneumoniae. Eur J Immunol. 1984;14:1027–1030. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases