Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties - PubMed (original) (raw)
Involvement of the extracellular signal-regulated kinase cascade for cocaine-rewarding properties
E Valjent et al. J Neurosci. 2000.
Abstract
A central feature of drugs of abuse is to induce gene expression in discrete brain structures that are critically involved in behavioral responses related to addictive processes. Although extracellular signal-regulated kinase (ERK) has been implicated in several neurobiological processes, including neuronal plasticity, its role in drug addiction remains poorly understood. This study was designed to analyze the activation of ERK by cocaine, its involvement in cocaine-induced early and long-term behavioral effects, as well as in gene expression. We show, by immunocytochemistry, that acute cocaine administration activates ERK throughout the striatum, rapidly but transiently. This activation was blocked when SCH 23390 [a specific dopamine (DA)-D1 antagonist] but not raclopride (a DA-D2 antagonist) was injected before cocaine. Glutamate receptors of NMDA subtypes also participated in ERK activation, as shown after injection of the NMDA receptor antagonist MK 801. The systemic injection of SL327, a selective inhibitor of the ERK kinase MEK, before cocaine, abolished the cocaine-induced ERK activation and decreased cocaine-induced hyperlocomotion, indicating a role of this pathway in events underlying early behavioral responses. Moreover, the rewarding effects of cocaine were abolished by SL327 in the place-conditioning paradigm. Because SL327 antagonized cocaine-induced c-fos expression and Elk-1 hyperphosphorylation, we suggest that the ERK intracellular signaling cascade is also involved in the prime burst of gene expression underlying long-term behavioral changes induced by cocaine. Altogether, these results reveal a new mechanism to explain behavioral responses of cocaine related to its addictive properties.
Figures
Fig. 1.
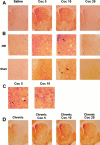
Acute cocaine injection activates ERK in the striatum. Immunocytochemical detection of activated ERK proteins was detected using a specific anti-active antibody, 5 (Coc 5), 10 (Coc 10), and 20 (Coc 20) min after cocaine injection (20 mg/kg, i.p.). A, Low magnification (50×) showing P-ERK immunoreactivity in the whole striatum. Note the strong immunoreactivity at Coc 5 and Coc 10 in the whole striatum and its decrease at Coc 20 (data are representative of 5 independent animals in each group). B, Higher magnification (200×) of P-ERK immunoreactivity in the dorsomedial striatum (DM) and the shell of NA. In both striatal regions, this immunoreactivity corresponds to both neuropil (asterisk) and cell bodies (arrows).C, Whereas at Coc 5 most of cell bodies showed a cytoplasmic (black arrows) P-ERK immunolabeling, at Coc 10, the majority of P-ERK was nuclear (white arrows).D, P-ERK immunoreactivity at low magnification showing ERK activation in chronically treated mice (6 d, once daily) with a challenge of cocaine (Coc 5, Coc 10, Coc 20) performed on day 7 (data are representative of 4 independent animals in each group).
Fig. 2.
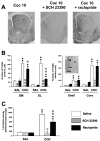
Cocaine-induced ERK activation depends on DA-D1 receptors. SCH 23390 (0.1 mg/kg, i.p.) or raclopride (0.3 mg/kg, i.p.) were injected 15 min before cocaine. A, Low magnification of P-ERK immunolabeling in the striatum in cocaine-injected mice, in the presence or not of DA antagonists. Note the total inhibition of cocaine-induced P-ERK immunolabeling by DA-D1 antagonist. B, P-ERK-immunoreactive cells were counted in the dorsomedial (DM) and dorsolateral (DL) striatum, core, and shell of the NA from three independent mice in each group. Data represents the total number of P-ERK-positive cells in each region, delimited as depicted in_insert._ C, The same doses of DA antagonists were used to analyze locomotor response induced by cocaine (n = 8 mice for each group). Statistical comparisons for B and C: ★p < 0.05; ★★ p < 0.001 when comparing with saline group. ⋆ p < 0.05; ⋆⋆ p < 0.001 when comparing with cocaine group (Newman–Keuls test).
Fig. 3.
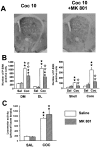
Partial contribution of glutamatergic NMDA receptors on cocaine-induced ERK activation_._ MK 801 (0.15 mg/kg, i.p.) was injected 30 min before cocaine.A, Low magnification of P-ERK immunolabeling in the striatum in cocaine-injected mice, in the presence or not of MK 801. Note the slight decrease of cocaine-induced P-ERK immunolabeling.B, P-ERK-immunoreactive cells were counted in the four striatal subregions as described Figure 3 (n = 3 independent mice for each group). C, The same dose of MK 801 was used to analyze locomotor response induced by cocaine (n = 8 mice for each group). Statistical comparisons for B and C were performed as described Figure 2.
Fig. 4.
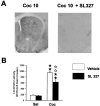
SL 327, an inhibitor of ERK activation, partially inhibits cocaine-induced hyperlocomotion_._ SL 327 (50 mg/kg, i.p.) was injected 1 hr before cocaine. A, Note the total inhibition of cocaine-induced P-ERK immunoreactivity by SL 327. B, Effect of SL 327 on cocaine-induced hyperlocomotion (n = 8 mice for each group). Statistical comparisons were performed as described Figure 2.
Fig. 5.
SL 327 inhibits c-fos induction by cocaine. c-fos expression was analyzed 1 hr after cocaine injection in the dorsomedial striatum (DM) and in the shell of the NA. SL 327 was injected as described Figure 4. Note the total inhibition of c-fos immunoreactivity in the DM and the strong but not total inhibition in the shell (data are representative of 4 independent mice for each group).
Fig. 6.
Cocaine-induced Elk-1 hyperphosphorylation depends on ERK activation. Elk-1 hyperphosphorylation was analyzed using an anti-active antibody (anti phospho-Ser383-Elk-1) 10 min after cocaine injection in the absence or presence of SL 327. Note the strong induction of Elk-1 activation by cocaine in the dorsomedial striatum (DM) and in the shell of the NA. This activation is totally inhibited by SL 327 (data are representative of 4 independent mice for each group).
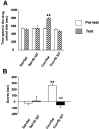
Fig. 7.
Lack of cocaine-induced place preference in mice injected with SL 327. A, Time spent in drug-associated compartment during the preconditioning (white bars) and the testing phase (hatched bars). Values represent mean ± SEM from n = 8 independent mice per group. B, Scores calculated as the difference between postconditioning and preconditioning time spent in the compartment associated with cocaine. ★★ p < 0.001 when comparing with saline group. ⋆⋆ p < 0.001 when comparing with cocaine group (Newman–Keuls test).
Similar articles
- Delta 9-tetrahydrocannabinol-induced MAPK/ERK and Elk-1 activation in vivo depends on dopaminergic transmission.
Valjent E, Pagès C, Rogard M, Besson MJ, Maldonado R, Caboche J. Valjent E, et al. Eur J Neurosci. 2001 Jul;14(2):342-52. doi: 10.1046/j.0953-816x.2001.01652.x. Eur J Neurosci. 2001. PMID: 11553284 - Dopamine induces a PI3-kinase-independent activation of Akt in striatal neurons: a new route to cAMP response element-binding protein phosphorylation.
Brami-Cherrier K, Valjent E, Garcia M, Pagès C, Hipskind RA, Caboche J. Brami-Cherrier K, et al. J Neurosci. 2002 Oct 15;22(20):8911-21. doi: 10.1523/JNEUROSCI.22-20-08911.2002. J Neurosci. 2002. PMID: 12388598 Free PMC article. - Dopamine D1 receptor, but not dopamine D2 receptor, is a critical regulator for acute cocaine-enhanced gene expression.
Guan X, Tao J, Li S. Guan X, et al. Neurol Res. 2009 Feb;31(1):17-22. doi: 10.1179/174313208X332986. Neurol Res. 2009. PMID: 19228458 - Extracellular signal-regulated protein kinases 1 and 2 activation by addictive drugs: a signal toward pathological adaptation.
Pascoli V, Cahill E, Bellivier F, Caboche J, Vanhoutte P. Pascoli V, et al. Biol Psychiatry. 2014 Dec 15;76(12):917-26. doi: 10.1016/j.biopsych.2014.04.005. Epub 2014 Apr 18. Biol Psychiatry. 2014. PMID: 24844603 Review. - Drug-induced alterations in the extracellular signal-regulated kinase (ERK) signalling pathway: implications for reinforcement and reinstatement.
Zhai H, Li Y, Wang X, Lu L. Zhai H, et al. Cell Mol Neurobiol. 2008 Feb;28(2):157-72. doi: 10.1007/s10571-007-9240-3. Epub 2007 Nov 28. Cell Mol Neurobiol. 2008. PMID: 18041576 Free PMC article. Review.
Cited by
- L-type Ca2+ channels mediate adaptation of extracellular signal-regulated kinase 1/2 phosphorylation in the ventral tegmental area after chronic amphetamine treatment.
Rajadhyaksha A, Husson I, Satpute SS, Küppenbender KD, Ren JQ, Guerriero RM, Standaert DG, Kosofsky BE. Rajadhyaksha A, et al. J Neurosci. 2004 Aug 25;24(34):7464-76. doi: 10.1523/JNEUROSCI.0612-04.2004. J Neurosci. 2004. PMID: 15329393 Free PMC article. - Signaling from the cytoplasm to the nucleus in striatal medium-sized spiny neurons.
Matamales M, Girault JA. Matamales M, et al. Front Neuroanat. 2011 Jul 6;5:37. doi: 10.3389/fnana.2011.00037. eCollection 2011. Front Neuroanat. 2011. PMID: 21779236 Free PMC article. - Relevance of ERK1/2 Post-retrieval Participation on Memory Processes: Insights in Their Particular Role on Reconsolidation and Persistence of Memories.
Krawczyk MC, Millan J, Blake MG, Feld M, Boccia MM. Krawczyk MC, et al. Front Mol Neurosci. 2019 Apr 17;12:95. doi: 10.3389/fnmol.2019.00095. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31057366 Free PMC article. Review. - The involvement of type IV phosphodiesterases in cocaine-induced sensitization and subsequent pERK expression in the mouse nucleus accumbens.
Janes AC, Kantak KM, Cherry JA. Janes AC, et al. Psychopharmacology (Berl). 2009 Oct;206(2):177-85. doi: 10.1007/s00213-009-1594-4. Epub 2009 Jul 9. Psychopharmacology (Berl). 2009. PMID: 19588125 - Inhibition of basal and amphetamine-stimulated extracellular signal-regulated kinase (ERK) phosphorylation in the rat forebrain by muscarinic acetylcholine M4 receptors.
He N, Mao LM, Sturich AW, Jin DZ, Wang JQ. He N, et al. Brain Res. 2018 Jun 1;1688:103-112. doi: 10.1016/j.brainres.2018.03.019. Epub 2018 Mar 22. Brain Res. 2018. PMID: 29577888 Free PMC article.
References
- Aizman O, Brismar H, Uhlen P, Zettergren E, Levey AI, Forssberg H, Greengard P, Aperia A. Anatomical and physiological evidence for D1 and D2 dopamine receptor colocalization in neostriatal neurons. Nat Neurosci. 2000;3:226–230. - PubMed
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. - PubMed
- Berke JD, Hyman SE. Addiction, dopamine, the molecular mechanisms of memory. Neuron. 2000;25:515–532. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous