Functional MR imaging in Alzheimer's disease during memory encoding - PubMed (original) (raw)
. 2000 Nov-Dec;21(10):1869-75.
Affiliations
- PMID: 11110539
- PMCID: PMC7974309
Functional MR imaging in Alzheimer's disease during memory encoding
S A Rombouts et al. AJNR Am J Neuroradiol. 2000 Nov-Dec.
Abstract
Background and purpose: We applied functional MR imaging with a learning task in healthy elderly volunteers and in patients with Alzheimer's disease to study brain activation during memory performance. The purpose was to determine the feasibility of functional MR imaging during a learning task in healthy elderly volunteers and in patients with Alzheimer's disease and to test our hypothesis that brain activation is decreased in the medial temporal lobe (MTL) memory system in patients with Alzheimer's disease compared with control volunteers.
Methods: In 12 patients with mild to moderate forms of Alzheimer's disease and 10 elderly control volunteers, activation of the MTL memory system was studied. We used two learning tasks that required the encoding of new information into memory. After the functional MR imaging experiment, participants were tested for recognition of the encoded objects.
Results: In the elderly control volunteers, activation during memory encoding was observed in medial and lateral temporal lobe structures (fusiform, parietal and occipital parts, and hippocampal formation) and in the frontal cortex, as reported previously in studies of young control volunteers. Focusing on the MTL, we observed that activation was significantly decreased in patients with Alzheimer's disease compared with control volunteers in the left hippocampus and parahippocampal gyrus bilaterally during the first encoding task but not during the second (P < .05, uncorrected).
Conclusion: Functional MR imaging with a learning task seems feasible in elderly volunteers and in patients with Alzheimer's disease. The measured functional signal decrease in MTL areas warrants further exploration of the (early) diagnostic usefulness of functional MR imaging in cases of Alzheimer's disease and other dementias.
Figures
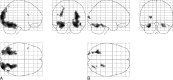
fig 1.
Projection of average brain activation during encoding of color pictures in sagittal, coronal, and transverse directions in the control group and in the patients. A, Control group (n = 10, left in picture is left in brain). A one-sample t test random effects analysis (P < .001; uncorrected; minimal cluster size, 108 mm3) was applied. Significant activation is observed in the occipital cortex, fusiform gyri, left parahippocampal gyrus, parietal lobe, and left inferior frontal gyrus. B, Patients (n = 11). The main effect of signal increase is seen in the occipital cortex, right parahippocampal gyrus, fusiform gyrus, and cerebellum.
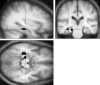
fig 2.
Sagittal, coronal, and transverse sections showing a significant increase in brain activation in control volunteers compared with patients in the left hippocampus and parahippocampal gyrus (black areas) during the first task, after application of the region of interest analysis (P < .05, uncorrected). The same effect is seen in the right parahippocampal gyrus (not shown). Activation is projected on the average brain of the 10 control volunteers (3D gradient-echo, T1-weighted sequence with parameters 15/7/1). Left in the figure is left in the brain
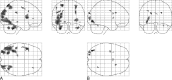
fig 3.
Projection of average brain activation during encoding of line drawings in sagittal, coronal, and transverse directions in the control group and in patients. A, Control group (n = 10, left in the figure is left in the brain). A one-sample t test random effects analysis (P < .001; uncorrected; minimal cluster size, 108 mm3) was applied. Significant activation is observed in the cerebellum, occipital cortex, parietal cortex, inferior frontal gyrus, and left parahippocampal gyrus. B, Patients (n = 8). The main effect of signal increase is seen in the occipital and parietal cortex, right precentral gyrus, left insula, left middle frontal gyrus, and cingulate sulcus.
Similar articles
- The neural correlates of verbal short-term memory in Alzheimer's disease: an fMRI study.
Peters F, Collette F, Degueldre C, Sterpenich V, Majerus S, Salmon E. Peters F, et al. Brain. 2009 Jul;132(Pt 7):1833-46. doi: 10.1093/brain/awp075. Epub 2009 May 11. Brain. 2009. PMID: 19433442 - Memory encoding in Alzheimer's disease: an fMRI study of explicit and implicit memory.
Golby A, Silverberg G, Race E, Gabrieli S, O'Shea J, Knierim K, Stebbins G, Gabrieli J. Golby A, et al. Brain. 2005 Apr;128(Pt 4):773-87. doi: 10.1093/brain/awh400. Epub 2005 Feb 10. Brain. 2005. PMID: 15705615 - Altered brain functional connectivity and impaired short-term memory in Alzheimer's disease.
Grady CL, Furey ML, Pietrini P, Horwitz B, Rapoport SI. Grady CL, et al. Brain. 2001 Apr;124(Pt 4):739-56. doi: 10.1093/brain/124.4.739. Brain. 2001. PMID: 11287374 Clinical Trial. - Lateralized anterior mesiotemporal lobe activation: semirandom functional MR imaging encoding paradigm in patients with temporal lobe epilepsy--initial experience.
Deblaere K, Backes WH, Tieleman A, Vandemaele P, Defreyne L, Vonck K, Hofman P, Boon P, Vermeulen J, Wilmink J, Aldenkamp A, Boon PA, Vingerhoets G, Achten E. Deblaere K, et al. Radiology. 2005 Sep;236(3):996-1003. doi: 10.1148/radiol.2363040780. Radiology. 2005. PMID: 16118173 - Verbal episodic memory impairment in Alzheimer's disease: a combined structural and functional MRI study.
Rémy F, Mirrashed F, Campbell B, Richter W. Rémy F, et al. Neuroimage. 2005 Mar;25(1):253-66. doi: 10.1016/j.neuroimage.2004.10.045. Epub 2004 Dec 10. Neuroimage. 2005. PMID: 15734360
Cited by
- Pattern separation and pattern completion in Alzheimer's disease: evidence of rapid forgetting in amnestic mild cognitive impairment.
Ally BA, Hussey EP, Ko PC, Molitor RJ. Ally BA, et al. Hippocampus. 2013 Dec;23(12):1246-58. doi: 10.1002/hipo.22162. Epub 2013 Aug 14. Hippocampus. 2013. PMID: 23804525 Free PMC article. - Advances in functional magnetic resonance imaging: technology and clinical applications.
Dickerson BC. Dickerson BC. Neurotherapeutics. 2007 Jul;4(3):360-70. doi: 10.1016/j.nurt.2007.05.007. Neurotherapeutics. 2007. PMID: 17599702 Free PMC article. Review. - Estimating effective connectivity in Alzheimer's disease progression: A dynamic causal modeling study.
Huang J, Jung JY, Nam CS. Huang J, et al. Front Hum Neurosci. 2022 Dec 15;16:1060936. doi: 10.3389/fnhum.2022.1060936. eCollection 2022. Front Hum Neurosci. 2022. PMID: 36590062 Free PMC article. - Medial temporal lobe function and structure in mild cognitive impairment.
Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Dickerson BC, et al. Ann Neurol. 2004 Jul;56(1):27-35. doi: 10.1002/ana.20163. Ann Neurol. 2004. PMID: 15236399 Free PMC article. - Quantitative magnetic resonance techniques as surrogate markers of Alzheimer's disease.
Kantarci K, Jack CR Jr. Kantarci K, et al. NeuroRx. 2004 Apr;1(2):196-205. doi: 10.1602/neurorx.1.2.196. NeuroRx. 2004. PMID: 15717020 Free PMC article. Review.
References
- Flicker C, Ferris SH, Reisberg B. Mild cognitive impairment in the elderly: predictors of dementia. Neurology 1991;41:1006-1009 - PubMed
- Braak H, Braak E. The human entorhinal cortex: normal morphology and lamina-specific pathology in various diseases. Neurosci Res 1992;15:6-31 - PubMed
- Fox NC, Warrington EK, Freeborough PA, et al. Presymptomatic hippocampal atrophy in Alzheimer's disease: a longitudinal MRI study. Brain 1996;119:2001-2007 - PubMed
- Jack CRJ, Petersen RC, O'Brien PC, Tangalos EG. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology 1992;42:183-188 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical