Influenza A virus NS2 protein mediates vRNP nuclear export through NES-independent interaction with hCRM1 - PubMed (original) (raw)
Influenza A virus NS2 protein mediates vRNP nuclear export through NES-independent interaction with hCRM1
G Neumann et al. EMBO J. 2000.
Abstract
For nuclear export of proteins, the formation of a ternary export complex composed of the export substrate, a cellular export factor and Ran-GTP is crucial. CRM1 is a cellular export factor for proteins containing leucine-rich nuclear export signals (NESs). Although the NES sequence is crucial for nuclear export, its exact role in the formation of the ternary export complex is controversial. Here we demonstrate an interaction between human CRM1 (hCRM1) and influenza A virus NS2 protein, which contains an NES motif in its N-terminal region. Replacement of the hydrophobic amino acids in the NES motif did not abolish NS2's interaction with hCRM1. Using our recently established systems for the generation of influenza virus or virus-like particles from cloned cDNAs, we found that NS2 is essential for nuclear export of influenza virus ribonucleoprotein (RNP) complexes, and that alteration of the NS2-NES abrogated this event and influenza virus generation. These findings suggest that the NS2-NES is not crucial for the interaction of this protein with hCRM1, but is for the formation of the ternary export complex with Ran-GTP.
Figures
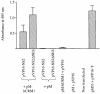
Fig. 1. Interaction of NS2 or its mutant with hCRM1 in the mammalian two-hybrid assay. 293T cells were cotransfected with the reporter gene construct pG5CAT, pM-hCRM1 (hCRM1 fused to the Gal4 DNA-binding domain) and wild-type or mutant NS2, fused to the HSV 16 activation domain (pVP16-NS2 or pVP16-NS2ΔNES). Negative controls included non-transfected cells, and cells transfected with the reporter gene and pVP16 + pM-hCRM1, pVP16-NS2 or pVP16-NS2ΔNES + pM, or pVP16 + pM. As a positive control, we determined the interaction between p53, fused to the Gal4-binding domain (pM53), and SV40 T-antigen, fused to the HSV 16 activation domain (pVP16-T). Forty-eight hours after transfection, CAT-ELISAs were performed. The bars denote the absorbance at 405 nm, which corresponds to the level of CAT expression and thus the level of interaction between hCRM1 and the protein of interest. The results are the mean ± SD for three experiments.
Fig. 2. (A) Schematic representation of RNA polymerase I constructs for NS vRNA synthesis. Solid bars represent NS vRNAs synthesized by RNA polymerase I. Translation products are shown as boxes (dotted boxes, NS1; striped boxes, NS2). pPolI-WSN-NSΔSplice: ‘ΔSplice Donor’ and ‘Stop’ indicate alteration of the splice donor sequence (G56A) and introduction of a stop signal at codon 17 in the NS2 reading frame, respectively. For all other constructs, ‘Stop’ indicates the introduction of two stop signals at codons 125 and 126 in the NS1 coding sequence. NES represents the leucine-rich motifs of NS2 (see below), whereas ΔNES indicates the altered export signal. (B) Amino acid sequences of NS2-NES and NS2ΔNES. To generate NS2ΔNES, we replaced the conserved hydrophobic amino acids with alanine, as indicated. The numbers indicate the amino acid position of A/WSN/33 NS2 protein.
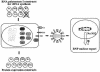
Fig. 3. Schematic diagram of a system for the analysis of the role of NS2 in NP nuclear export, using VLPs. 293T cells were transfected with plasmids expressing all viral structural proteins and seven RNA polymerase I plasmids for vRNA synthesis, omitting pPolI-WSN-NS. Forty-eight hours after transfection, supernatants derived from transfected cells were used to infect MDCK cells. Cells were fixed at 24 h p.i. and processed for indirect immunofluorescence assays, using antibodies against NP and NS2 to evaluate the effects of NS2 on RNP nuclear export.
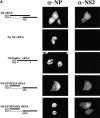
Fig. 4. Intracellular localization of NP and NS2 (A), or NP and M1 (B). MDCK cells were infected with VLPs, and immunofluorescence assays were performed 24 h p.i. NP localization was assessed with an anti-NP monoclonal antibody. NS2 and M1 were detected with rabbit antisera against these proteins.
Fig. 4. Intracellular localization of NP and NS2 (A), or NP and M1 (B). MDCK cells were infected with VLPs, and immunofluorescence assays were performed 24 h p.i. NP localization was assessed with an anti-NP monoclonal antibody. NS2 and M1 were detected with rabbit antisera against these proteins.
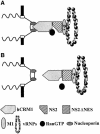
Fig. 5. A model for NS2-mediated vRNP nuclear export through the hCRM1 nuclear export pathway. (A) A ternary export complex is formed between hCRM1, Ran-GTP and NS2, which possibly interacts with vRNPs through M1. Interaction of the export complex with nucleoporins triggers nuclear export. (B) Replacement of the NS2-NES does not abrogate interaction with hCRM1, but does the formation of a stable ternary export complex and hence vRNP nuclear export, although experimental evidence is lacking.
Similar articles
- Generation of influenza A virus NS2 (NEP) mutants with an altered nuclear export signal sequence.
Iwatsuki-Horimoto K, Horimoto T, Fujii Y, Kawaoka Y. Iwatsuki-Horimoto K, et al. J Virol. 2004 Sep;78(18):10149-55. doi: 10.1128/JVI.78.18.10149-10155.2004. J Virol. 2004. PMID: 15331747 Free PMC article. - A supraphysiological nuclear export signal is required for parvovirus nuclear export.
Engelsma D, Valle N, Fish A, Salomé N, Almendral JM, Fornerod M. Engelsma D, et al. Mol Biol Cell. 2008 Jun;19(6):2544-52. doi: 10.1091/mbc.e08-01-0009. Epub 2008 Apr 2. Mol Biol Cell. 2008. PMID: 18385513 Free PMC article. - CRM1 mediates nuclear export of nonstructural protein 2 from parvovirus minute virus of mice.
Ohshima T, Nakajima T, Oishi T, Imamoto N, Yoneda Y, Fukamizu A, Yagami Ki. Ohshima T, et al. Biochem Biophys Res Commun. 1999 Oct 14;264(1):144-50. doi: 10.1006/bbrc.1999.1478. Biochem Biophys Res Commun. 1999. PMID: 10527855 - Leucine-rich nuclear-export signals: born to be weak.
Kutay U, Güttinger S. Kutay U, et al. Trends Cell Biol. 2005 Mar;15(3):121-4. doi: 10.1016/j.tcb.2005.01.005. Trends Cell Biol. 2005. PMID: 15752974 Review. - Nucleocytoplasmic shuttling of influenza A virus proteins.
Li J, Yu M, Zheng W, Liu W. Li J, et al. Viruses. 2015 May 22;7(5):2668-82. doi: 10.3390/v7052668. Viruses. 2015. PMID: 26008706 Free PMC article. Review.
Cited by
- Efficient genome replication in influenza A virus requires NS2 and sequence beyond the canonical promoter.
Swaminath S, Mendes M, Zhang Y, Remick KA, Mejia I, Güereca M, Te Velthuis AJW, Russell AB. Swaminath S, et al. bioRxiv [Preprint]. 2024 Sep 10:2024.09.10.612348. doi: 10.1101/2024.09.10.612348. bioRxiv. 2024. PMID: 39314307 Free PMC article. Preprint. - ProcCluster® and procaine hydrochloride inhibit the replication of influenza A virus in vitro.
Häring C, Schroeder J, Jungwirth J, Löffler B, Henke A, Engert B, Ehrhardt C. Häring C, et al. Front Microbiol. 2024 Aug 14;15:1422651. doi: 10.3389/fmicb.2024.1422651. eCollection 2024. Front Microbiol. 2024. PMID: 39206370 Free PMC article. - Unveiling the Antiviral Potential of Minocycline: Modulation of Nuclear Export of Viral Ribonuclear Proteins during Influenza Virus Infection.
Saha P, Saha R, Datta Chaudhuri R, Sarkar R, Sarkar M, Koley H, Chawla-Sarkar M. Saha P, et al. Viruses. 2024 Aug 18;16(8):1317. doi: 10.3390/v16081317. Viruses. 2024. PMID: 39205291 Free PMC article. - NS2 induces an influenza A RNA polymerase hexamer and acts as a transcription to replication switch.
Sun J, Kuai L, Zhang L, Xie Y, Zhang Y, Li Y, Peng Q, Shao Y, Yang Q, Tian WX, Zhu J, Qi J, Shi Y, Deng T, Gao GF. Sun J, et al. EMBO Rep. 2024 Nov;25(11):4708-4727. doi: 10.1038/s44319-024-00208-4. Epub 2024 Jul 18. EMBO Rep. 2024. PMID: 39026012 Free PMC article. - OTUB1 contributes to the stability and function of Influenza A virus NS2.
Li YJ, Chen CY, Kuo YS, Huang YW, Kuo RL, Chang LK, Yang JH, Lai CH, Shih SR, Chiu YF. Li YJ, et al. PLoS Pathog. 2024 May 30;20(5):e1012279. doi: 10.1371/journal.ppat.1012279. eCollection 2024 May. PLoS Pathog. 2024. PMID: 38814988 Free PMC article.
References
- Askjaer P., Jensen,T.H., Nilsson,J., Englmeier,L. and Kjems,J. (1998) The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J. Biol. Chem., 273, 33414–33422. - PubMed
- Bischoff F.R. and Ponstingl,H. (1991) Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature, 354, 80–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous