Context-dependent anticodon recognition by class I lysyl-tRNA synthetases - PubMed (original) (raw)
Context-dependent anticodon recognition by class I lysyl-tRNA synthetases
D Söll et al. Proc Natl Acad Sci U S A. 2000.
Abstract
Lysyl-tRNA synthesis is catalyzed by two unrelated families of aminoacyl-tRNA synthetases. In most bacteria and all eukarya, the known lysyl-tRNA synthetases (LysRSs) are subclass IIb-type aminoacyl-tRNA synthetases, whereas many archaea and a scattering of bacteria contain an unrelated class I-type LysRS. Examination of the recognition of partially modified tRNA(Lys) anticodon variants by a bacterial (from Borrelia burgdorferi) and an archaeal (from Methanococcus maripaludis) class I lysyl-tRNA synthetase revealed differences in the pattern of anticodon recognition between the two enzymes. U35 and U36 were both important for recognition by the B. burgdorferi enzyme, whereas only U36 played a role in recognition by M. maripaludis LysRS. Examination of the phylogenetic distribution of class I LysRSs suggested a correlation between recognition of U35 and U36 and the presence of asparaginyl-tRNA synthetase (AsnRS), which also recognizes U35 and U36 in the anticodon of tRNA(Asn). However, the class II LysRS of Helicobacter pylori, an organism that lacks AsnRS, also recognizes both U35 and U36, indicating that the presence of AsnRS has solely influenced the phylogenetic distribution of class I LysRSs. These data suggest that competition between unrelated aminoacyl-tRNA synthetases for overlapping anticodon sequences is a determinant of the phylogenetic distribution of extant synthetase families. Such patterns of competition also provide a basis for the two separate horizontal gene transfer events hypothesized in the evolution of the class I lysyl-tRNA synthetases.
Figures
Figure 1
Secondary structure of wild-type E. coli tRNALys. D, dihydrouridine; mnm5s2U, 5-methylaminomethyl-2-thiouridine; t6A,_N_6-threonylcarbamoyladenosine; ψ, pseudouridine; m7G, 7-methylguanosine; X, putative 3-(3-amino-3-carboxypropyl) uridine modification. The undermodified variants used in this study contain U34 in place of mnm5s2U34 and A37 in place of t6A37.
Figure 2
Aminoacylation of E. coli tRNALys variants by M. maripaludis His6-LysRS. Aminoacylation reactions were performed as described (20 μl samples) in the presence of 200 nM enzyme, 8 μM tRNA, and 500 μM [3H] lysine. Comparison of charging of wild-type tRNA (UUU anticodon) to variants of: (A) U34; (B) U35; (C) U36.
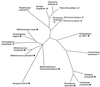
Figure 3
Unrooted phylogeny of class I LysRS proteins. The phylogeny was constructed by using the maximum likelihood method (5,000 puzzling steps) implemented in the program TREE-PUZZLE 4.0.2 under the JTT model. ✖, genome does not encode AsnRS; √, genome encodes AsnRS; ?, genome sequence incomplete, AsnRS encoding gene not yet detected.
References
- Ibba M, Söll D. Annu Rev Biochem. 2000;69:617–650. - PubMed
- Ibba M, Becker H D, Stathopoulos C, Tumbula D L, Söll D. Trends Biochem Sci. 2000;25:311–316. - PubMed
- Tumbula D L, Becker H D, Chang W-Z, Söll D. Nature (London) 2000;407:106–110. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources