Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood - PubMed (original) (raw)
Mosaic blood vessels in tumors: frequency of cancer cells in contact with flowing blood
Y S Chang et al. Proc Natl Acad Sci U S A. 2000.
Abstract
The presence of "mosaic" vessels in which both endothelial cells and tumor cells form the luminal surface has profound implications for metastasis, drug delivery, and antivascular therapy. Yet little is known of the frequency, and thus importance, of mosaic vessels in tumors. Using CD31 and CD105 to identify endothelial cells and endogenous green fluorescent protein labeling of tumor cells, we show that approximately 15% of perfused vessels of a colon carcinoma xenografted at two different sites in mice were mosaic vessels having focal regions where no CD31/CD105 immunoreactivity was detected and tumor cells appeared to contact the vessel lumen. These regions occupied approximately 25% of the perimeter of the mosaic vessels, or approximately 4% of the total vascular surface area in these colon carcinomas. In addition, we found similar numbers of mosaic vessels in human colon carcinoma biopsies. Our results are consistent with the observation that approximately 10(6) cells are shed daily per g of tumor. More importantly, our data offer a possible explanation for the antivascular effects of cytotoxic agents and suggest potential strategies for targeting the tumor vasculature.
Figures
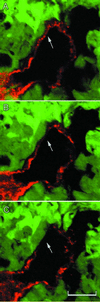
Figure 1
Identification of cellular constituents of the vascular wall in LS174T tumor xenografts. (A) Summary of the method used to identify mosaic vessels. Endogenous GFP expression by the implanted tumor cells (green) along with lectin fluorescence to mark perfused vessels (blue, rhodamine pseudocolor) and CD31/CD105 immunostaining (red, Cy5) for endothelium defines the cellular composition of the tumor vessels. (B) Composite three-color representation of the vessel in A. (C and D) Individual confocal slices (1 μm) from the orthotopic (C) and the ectopic tumors (D) showing apparent tumor cell involvement in the wall. The lectin staining has been omitted for clarity. Arrows mark regions that lack detectable CD31/CD105 immunoreactivity. (Scale bar:A and C, 20 μm; D, 50 μm.)
Figure 2
Confocal slices from three different depths within one of the orthotopic tumors. C shows a region where the red CD31/CD105 immunoreactivity is no longer visible. (Scale bar, 20 μm; depth between each section, 2.5 μm.)
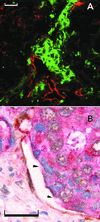
Figure 3
Mosaic vessels in human colon cancer. (A) Fluorescence confocal image of double-staining with anti-CD31 (visualized with Texas red), anticarcinoembryonic antigen (visualized with FITC). Arrowhead marks a region lacking detectable CD31 immunoreactivity. (B) Bright-field double-staining with anti-CD31 (visualized with diaminobenzidine), anticarcinoembryonic antigen (visualized with fast-red), and hematoxylin. (Scale bars: 50 μm.)
Figure 4
Potential mechanisms of mosaic vessel formation (endothelial cells: red; cancer cells: blue). In A, rapid vessel growth occurs via endothelial migration without sufficient endothelial proliferation, leaving cancer cells (dark blue) exposed to the lumen. In B, an endothelial cell (red) is shed from the lining, exposing underlying tumor cell(s). In C, a tumor cell (dark blue) invades the vessel, displacing an endothelial cell that may later be lost from the lining. Our morphological data are most consistent with B.
Comment in
- Can mosaic tumor vessels facilitate molecular diagnosis of cancer?
Folkman J. Folkman J. Proc Natl Acad Sci U S A. 2001 Jan 16;98(2):398-400. doi: 10.1073/pnas.98.2.398. Proc Natl Acad Sci U S A. 2001. PMID: 11209044 Free PMC article. No abstract available.
Similar articles
- Mosaic tumor vessels: cellular basis and ultrastructure of focal regions lacking endothelial cell markers.
di Tomaso E, Capen D, Haskell A, Hart J, Logie JJ, Jain RK, McDonald DM, Jones R, Munn LL. di Tomaso E, et al. Cancer Res. 2005 Jul 1;65(13):5740-9. doi: 10.1158/0008-5472.CAN-04-4552. Cancer Res. 2005. PMID: 15994949 - Endoglin (CD105) expression in angiogenesis of colon cancer: analysis using tissue microarrays and comparison with other endothelial markers.
Minhajat R, Mori D, Yamasaki F, Sugita Y, Satoh T, Tokunaga O. Minhajat R, et al. Virchows Arch. 2006 Feb;448(2):127-34. doi: 10.1007/s00428-005-0062-8. Epub 2005 Sep 22. Virchows Arch. 2006. PMID: 16177881 - CD105 is important for angiogenesis: evidence and potential applications.
Duff SE, Li C, Garland JM, Kumar S. Duff SE, et al. FASEB J. 2003 Jun;17(9):984-92. doi: 10.1096/fj.02-0634rev. FASEB J. 2003. PMID: 12773481 Review. - Endoglin (CD105): a powerful therapeutic target on tumor-associated angiogenetic blood vessels.
Fonsatti E, Altomonte M, Nicotra MR, Natali PG, Maio M. Fonsatti E, et al. Oncogene. 2003 Sep 29;22(42):6557-63. doi: 10.1038/sj.onc.1206813. Oncogene. 2003. PMID: 14528280 Review.
Cited by
- The pre-metastatic niche: is metastasis random?
Cox TR, Gartland A, Erler JT. Cox TR, et al. Bonekey Rep. 2012 May 2;1:80. doi: 10.1038/bonekey.2012.80. eCollection 2012. Bonekey Rep. 2012. PMID: 27127624 Free PMC article. Review. - Surfactant functionalization induces robust, differential adhesion of tumor cells and blood cells to charged nanotube-coated biomaterials under flow.
Mitchell MJ, Castellanos CA, King MR. Mitchell MJ, et al. Biomaterials. 2015 Jul;56:179-86. doi: 10.1016/j.biomaterials.2015.03.045. Epub 2015 Apr 17. Biomaterials. 2015. PMID: 25934290 Free PMC article. - Prognostic significance and mechanisms of patterned matrix vasculogenic mimicry in hepatocellular carcinoma.
Liu WB, Xu GL, Jia WD, Li JS, Ma JL, Chen K, Wang ZH, Ge YS, Ren WH, Yu JH, Wang W, Wang XJ. Liu WB, et al. Med Oncol. 2011 Dec;28 Suppl 1:S228-38. doi: 10.1007/s12032-010-9706-x. Epub 2010 Oct 19. Med Oncol. 2011. PMID: 20957524 - Vasculogenic Mimicry in Head and Neck Squamous Cell Carcinoma-Time to Take Notice.
Salem A, Salo T. Salem A, et al. Front Oral Health. 2021 Mar 31;2:666895. doi: 10.3389/froh.2021.666895. eCollection 2021. Front Oral Health. 2021. PMID: 35048009 Free PMC article. Review. - Therapeutic Effects of Cold Atmospheric Plasma on Solid Tumor.
Min T, Xie X, Ren K, Sun T, Wang H, Dang C, Zhang H. Min T, et al. Front Med (Lausanne). 2022 May 13;9:884887. doi: 10.3389/fmed.2022.884887. eCollection 2022. Front Med (Lausanne). 2022. PMID: 35646968 Free PMC article. Review.
References
- Willis R A. Pathology of Tumours. London: Butterworth; 1948.
- Warren B A, Shubik P. Lab Invest. 1966;15:464–478. - PubMed
- Prause J U, Jensen O A. Albrecht Von Graefes Arch Klin Exp Ophthalmol. 1980;212:261–270. - PubMed
- Hammersen F, Endrich B, Messmer K. Int J Microcirc Clin Exp. 1985;4:31–43. - PubMed
- Jain R K. Cancer Res. 1988;48:2641–2658. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 CA073479/CA/NCI NIH HHS/United States
- P01 HL024136/HL/NHLBI NIH HHS/United States
- R35 CA 56591/CA/NCI NIH HHS/United States
- P01 CA080124/CA/NCI NIH HHS/United States
- P01 CA80124/CA/NCI NIH HHS/United States
- T32 CA-73479/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources