MAP1B is required for axon guidance and Is involved in the development of the central and peripheral nervous system - PubMed (original) (raw)
MAP1B is required for axon guidance and Is involved in the development of the central and peripheral nervous system
A Meixner et al. J Cell Biol. 2000.
Abstract
Microtubule-associated proteins such as MAP1B have long been suspected to play an important role in neuronal differentiation, but proof has been lacking. Previous MAP1B gene targeting studies yielded contradictory and inconclusive results and did not reveal MAP1B function. In contrast to two earlier efforts, we now describe generation of a complete MAP1B null allele. Mice heterozygous for this MAP1B deletion were not affected. Homozygous mutants were viable but displayed a striking developmental defect in the brain, the selective absence of the corpus callosum, and the concomitant formation of myelinated fiber bundles consisting of misguided cortical axons. In addition, peripheral nerves of MAP1B-deficient mice had a reduced number of large myelinated axons. The myelin sheaths of the remaining axons were of reduced thickness, resulting in a decrease of nerve conduction velocity in the adult sciatic nerve. On the other hand, the anticipated involvement of MAP1B in retinal development and gamma-aminobutyric acid C receptor clustering was not substantiated. Our results demonstrate an essential role of MAP1B in development and function of the nervous system and resolve a previous controversy over its importance.
Figures
Figure 1
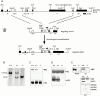
Deletion of 93% of the coding region of the mouse chromosomal MAP1B locus leads to MAP1B heavy- and light-chain deficiency. (A) Schematic of the targeting strategy. MAP1B coding exons present in regular transcripts are indicated by filled boxes, noncoding exons (IIIA and IIIU) present in alternative transcripts by open boxes. Exons are numbered (I–VII). Relevant restriction sites, ATG start codons for regular transcripts (exon I), and alternative transcripts (exons IV and V), the heavy chain–light chain cleavage site of the polyprotein precursor, the TGA stop codon, as well as the position of the exon VII probe are indicated. The EcoRV restriction sites of the wild-type and targeted alleles that were used for diagnostic digests are marked (*). The horizontal arrow above the neo cassette indicates the direction of its transcription. For better visibility, the small MAP1B exons and the neo cassette are drawn disproportionately long. (B) Southern blot analysis of tail DNA of wild-type (+/+), heterozygous (+/−), and homozygous (−/−) MAP1B mutant mice using EcoRV and the exon VII probe. The diagnostic EcoRV fragments of the wild-type and targeted allele are 11.5 and 9 kb, respectively. Immunoblot analysis of MAP1B expression. 80 μg of total protein extracted from brains of 1-d-old wild-type (+/+), heterozygous (+/−) or homozygous (−/−) MAP1B mutant mice were fractionated on 7.5% (C) or 12% (D) gels. Blots were incubated with monoclonal antibody MAP5 (C) or polyclonal anti–light-chain antibody (D) to detect heavy chain (HC) and light chain (LC1), respectively. Tubulin (Tb) detected on the same blots with antitubulin antibodies served as internal control. The LC1 doublet detected in wild-type and heterozygous mice was seen consistently and might be due to post-translational modification of the light chain. (E) Immunoblot analysis of expression of an amino-terminal MAP1B fragment potentially encoded by the targeted allele. 80 μg of total protein extracted from brains of 1-d-old wild-type (+/+) or homozygous (−/−) MAP1B mutant mice were fractionated on a 6% gel. Expression of the heavy chain (HC) was analyzed using the monoclonal antibody MAP5 and an affinity purified polyclonal rabbit antipeptide antibody (E2) directed against mouse MAP1B amino acids 45–58 encoded by exon 2 as indicated. Both antibodies detected a protein band of the same size in wild-type but not mutant mice, demonstrating that the antipeptide antibody E2 recognized and was specific for the MAP1B heavy chain. The same samples were fractionated on a 17.5% gel. The resulting blot was cut into three horizontal segments. The segment containing proteins above 46 kD in size was incubated with antitubulin antibodies for the detection of tubulin (Tb; internal control). The segment containing proteins from 20–46 kD was incubated with the polyclonal anti–MAP1B light chain antibody (LC1). The segment containing proteins smaller than 20 kD was incubated with antibody E2 as indicated for the detection of the 95 amino acid–long amino-terminal MAP1B fragment potentially encoded by exons 1 and 2 of the targeted allele with an expected size of 10 kD. No such amino-terminal fragment was detected.
Figure 2
Expression of brain-specific MAPs other than MAP1B in MAP1B-deficient mice. 80 μg of total protein extracted from brains of wild-type (+/+), heterozygous (+/−) or homozygous (−/−) MAP1B mutant mice of the indicated postnatal ages were analyzed by immunoblotting using antibodies specific for the respective proteins. Expression was not determined for MAP1A heavy chain at day 1 or MAP1A light chain at day 49.
Figure 5
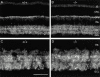
GABAC receptor clustering in MAP1B-deficient retina. (A) Section through a (+/+) mouse retina that was immunolabeled for the GABAC receptor subunits. (B) GABAC receptor immunolabeling in a(−/−) mouse retina. (C) High power micrograph of a (+/+) mouse retina showing the GABAC receptor synaptic clusters in the IPL. (D) GABAC receptor synaptic clusters in the IPL of a (−/−) mouse retina. IS, photoreceptor inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar: 55 μm (A and B) and 20 μm (C and D).
Figure 3
MAP1B-deficient mice lack exploring activity. Exploring activity of wild-type control mice (+/+) and mice homozygous (−/−) for the targeted MAP1B allele were tested for exploring activity.
Figure 4
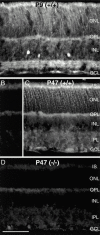
MAP1B expression in the mouse retina. (A) Fluorescence micrograph of a vertical section through a wild-type (+/+) mouse retina at postnatal day P9 that was immunostained for MAP1B. The inner plexiform layer is clearly labeled with a concentration of MAP1B in several discrete bands. MAP1B is also expressed in the outer retina. (B) Vertical section through an adult (+/+) mouse retina (postnatal day P47). In this control micrograph, the primary antibody against MAP1B was omitted and only nonspecific background label is detected. (C) Fluorescence micrograph of a vertical section through an adult (P47) wild-type (+/+) mouse retina that was immunostained for MAP1B. Diffuse, weak fluorescence is present in the inner retina, the outer retina is more intensely labeled. (D) Section through a MAP1B-deficient (−/−) mouse retina that was immunostained for MAP1B. No specific label can be detected, background intensity is identical to B. However, comparison of D and C shows that the retinal layers are unchanged in the MAP1B mutant mouse. IS, photoreceptor inner segments; ONL, outer nuclear layer; OPL, outer plexiform layer; INL, inner nuclear layer; GCL, ganglion cell layer. Bar, 50 μm.
Figure 6
Agenesis of the corpus callosum and formation of Probst bundles in the brain of MAP1B-deficient mice. (a, c, e, g, and i) Frontal sections through the cerebral white matter and corpus callosum (CC) of a normal wild-type mouse brain, showing normal architecture of myelinated fiber tracts including the corpus callosum (a, c, and e, blue), axons (g, dark brown), and astrocytes (i, brown). (b, d, f, h, and j) Corresponding brain region of a homozygous MAP1B-deficient mouse. The corpus callosum is absent. The cerebral white matter ends medially in a thick bundle of myelinated fibers (*Probst bundle). Within the deeper layers of the cortex, thick bundles of myelinated axons are visible (arrowheads). Despite the massive structural changes in the brain tissue, there is no increase in reactive astrocytes. CX, cerebral cortex. (a–d) Luxol fast blue myelin stain; myelinated fiber tracts such as the corpus callosum are stained blue, areas of grey matter are stained red, 40×. (e and f) Luxol fast blue myelin stain, 120×. (g and h) Bielschowski silver impregnation; axons within fiber tracts are stained black, areas of grey matter reveal light brown staining, 120×. (i and j) Immunocytochemistry for glial fibrillary acidic protein; astrocytes containing glial fibrillary acidic protein are stained brown. Cellular nuclei of the tissue are counterstained with hematoxylin blue, 120×.
Figure 7
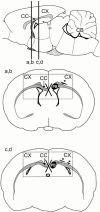
Schematic representation of brain areas shown in Fig. 6. (Top) Side view of the rodent brain (sagittal section). The locations of the frontal sections shown in Fig. 6, a and b and c and d, are indicated by vertical lines labeled a,b and c,d, respectively. CB, cerebellum. (Middle and bottom) Schematic representation of the frontal sections shown in Fig. 6, a and b (a,b) and c and d (c,d). The left side of the brain in each schematic shows the normal situation, the right side the changes in MAP1B-deficient animals. In the normal brain, nerve fibers insert into the corpus callosum (CC) and cross the midline. In MAP1B-deficient animals, these fibers do not cross the midline (indicated by the X), but form a thick bundle of fibers that runs in the longitudinal direction of the brain (PB, Probst bundle). In addition, thick aberrant fiber bundles leave the centrum semiovale and enter the basal layers of the cortex, where they turn by 90° and further traverse in parallel to the cortical surface (*). The rectangles in the middle and bottom panels delineate the areas shown in Fig. 6, a and b and c and d, respectively. CX, cerebral cortex.
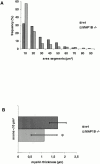
Figure 8
Predominance of small myelinated nerve fibers (A) and reduced thickness of myelin sheaths (B) in the sciatic nerve of MAP1B-deficient mice. Sciatic nerve preparations from three wild-type and four MAP1B-deficient mice were used to obtain the data. (A) The size distribution of axon areas differed significantly (Kolmogoroff-Smirnoff test, P < 0.001) between MAP1B-deficient (n = 465) and wild-type (n = 206) sciatic axons. (B) Myelin sheath thickness was assessed from axons with areas <10 μm (MAP1B −/−, n = 265, vs. wild-type, n = 66). *The mean values of the myelin sheath thickness were significantly smaller (t test, P < 0.001) in MAP1B −/− mice (1.11 ± 0.36 μm) compared with wild-type animals (1.70 ± 0.57 μm).
Similar articles
- Microtubule-associated protein 1B controls directionality of growth cone migration and axonal branching in regeneration of adult dorsal root ganglia neurons.
Bouquet C, Soares S, von Boxberg Y, Ravaille-Veron M, Propst F, Nothias F. Bouquet C, et al. J Neurosci. 2004 Aug 11;24(32):7204-13. doi: 10.1523/JNEUROSCI.2254-04.2004. J Neurosci. 2004. PMID: 15306655 Free PMC article. - Microtubule-associated protein 1B is involved in the initial stages of axonogenesis in peripheral nervous system cultured neurons.
Gonzalez-Billault C, Owen R, Gordon-Weeks PR, Avila J. Gonzalez-Billault C, et al. Brain Res. 2002 Jul 5;943(1):56-67. doi: 10.1016/s0006-8993(02)02534-9. Brain Res. 2002. PMID: 12088839 - Perinatal lethality of microtubule-associated protein 1B-deficient mice expressing alternative isoforms of the protein at low levels.
González-Billault C, Demandt E, Wandosell F, Torres M, Bonaldo P, Stoykova A, Chowdhury K, Gruss P, Avila J, Sánchez MP. González-Billault C, et al. Mol Cell Neurosci. 2000 Oct;16(4):408-21. doi: 10.1006/mcne.2000.0880. Mol Cell Neurosci. 2000. PMID: 11085878 - Applications of Proteomics to Nerve Regeneration Research.
Massing MW, Robinson GA, Marx CE, Alzate O, Madison RD. Massing MW, et al. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. In: Alzate O, editor. Neuroproteomics. Boca Raton (FL): CRC Press/Taylor & Francis; 2010. Chapter 15. PMID: 21882439 Free Books & Documents. Review. - MAP1B expression and microtubule stability in growing and regenerating axons.
Gordon-Weeks PR, Fischer I. Gordon-Weeks PR, et al. Microsc Res Tech. 2000 Jan 15;48(2):63-74. doi: 10.1002/(SICI)1097-0029(20000115)48:2<63::AID-JEMT2>3.0.CO;2-1. Microsc Res Tech. 2000. PMID: 10649507 Review.
Cited by
- Netrin-1 stimulated axon growth requires the polyglutamylase TTLL1.
Northington KR, Calderon J, Bates EA. Northington KR, et al. Front Neurosci. 2024 Oct 14;18:1436312. doi: 10.3389/fnins.2024.1436312. eCollection 2024. Front Neurosci. 2024. PMID: 39469034 Free PMC article. - Microtubule-associated protein, MAP1B, encodes functionally distinct polypeptides.
Tan TC, Shen Y, Stine LB, Mitchell B, Okada K, McKenney RJ, Ori-McKenney KM. Tan TC, et al. J Biol Chem. 2024 Sep 19;300(11):107792. doi: 10.1016/j.jbc.2024.107792. Online ahead of print. J Biol Chem. 2024. PMID: 39305956 Free PMC article. - Single-molecule live-cell RNA imaging with CRISPR-Csm.
Xia C, Colognori D, Jiang X, Xu K, Doudna JA. Xia C, et al. bioRxiv [Preprint]. 2024 Jul 16:2024.07.14.603457. doi: 10.1101/2024.07.14.603457. bioRxiv. 2024. PMID: 39071319 Free PMC article. Preprint. - RNA-binding proteins potentially regulate the alternative splicing of cell cycle-associated genes in proliferative diabetic retinopathy.
Yang N, Zhang N, Lu G, Zeng S, Xing Y, Du L. Yang N, et al. Sci Rep. 2024 Mar 20;14(1):6731. doi: 10.1038/s41598-024-57516-x. Sci Rep. 2024. PMID: 38509306 Free PMC article. - Targeted RNAseq Revealed the Gene Expression Signature of Ferroptosis-Related Processes Associated with Disease Severity in Patients with Multiple Sclerosis.
Stojkovic L, Jovanovic I, Dincic E, Djordjevic A, Kuveljic J, Djuric T, Stankovic A, Vojinovic S, Zivkovic M. Stojkovic L, et al. Int J Mol Sci. 2024 Mar 5;25(5):3016. doi: 10.3390/ijms25053016. Int J Mol Sci. 2024. PMID: 38474262 Free PMC article.
References
- Brugg B., Reddy D., Matus A. Attenuation of microtubule-associated protein 1B expression by antisense oligonucleotides inhibits initiation of neurite outgrowth. Neuroscience. 1993;52:489–496. - PubMed
- Bush M.S., Goold R.G., Moya F., Gordon-Weeks P.R. An analysis of an axonal gradient of phosphorylated MAP 1B in cultured rat sensory neurons Eur. J. Neurosci. 8 1996. 235 248a - PubMed
- Bush M.S., Tonge D.A., Woolf C., Gordon-Weeks P.R. Expression of a developmentally regulated, phosphorylated isoform of microtubule-associated protein 1B in regenerating axons of the sciatic nerve Neuroscience. 73 1996. 553 563b - PubMed
- DiTella M.C., Feiguin F., Carri N., Kosik K.S., Cáceres A. MAP-1B/TAU functional redundancy during laminin-enhanced axonal growth. J. Cell Sci. 1996;109:467–477. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases