Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells - PubMed (original) (raw)
Expression of CD34 and Myf5 defines the majority of quiescent adult skeletal muscle satellite cells
J R Beauchamp et al. J Cell Biol. 2000.
Abstract
Skeletal muscle is one of a several adult post-mitotic tissues that retain the capacity to regenerate. This relies on a population of quiescent precursors, termed satellite cells. Here we describe two novel markers of quiescent satellite cells: CD34, an established marker of hematopoietic stem cells, and Myf5, the earliest marker of myogenic commitment. CD34(+ve) myoblasts can be detected in proliferating C2C12 cultures. In differentiating cultures, CD34(+ve) cells do not fuse into myotubes, nor express MyoD. Using isolated myofibers as a model of synchronous precursor cell activation, we show that quiescent satellite cells express CD34. An early feature of their activation is alternate splicing followed by complete transcriptional shutdown of CD34. This data implicates CD34 in the maintenance of satellite cell quiescence. In heterozygous Myf5(nlacZ/+) mice, all CD34(+ve) satellite cells also express beta-galactosidase, a marker of activation of Myf5, showing that quiescent satellite cells are committed to myogenesis. All such cells are positive for the accepted satellite cell marker, M-cadherin. We also show that satellite cells can be identified on isolated myofibers of the myosin light chain 3F-nlacZ-2E mouse as those that do not express the transgene. The numbers of satellite cells detected in this way are significantly greater than those identified by the other three markers. We conclude that the expression of CD34, Myf5, and M-cadherin defines quiescent, committed precursors and speculate that the CD34(-ve), Myf5(-ve) minority may be involved in maintaining the lineage-committed majority.
Figures
Figure 1
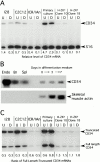
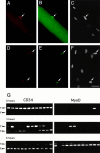
CD34 expression in primary muscle cultures and clonal myogenic cell lines. (A) RT-PCR analysis of CD34 expression in skeletal muscle cultures. Levels of CD34 mRNA in undifferentiated (U) and differentiated (D) cultures were calculated relative to transcript for S16 ribosomal protein. (B) Northern blot analysis of CD34 mRNA in differentiating C2C12 cultures. 30 μg of total RNA isolated from myoblasts (day 0) and from cultures induced to differentiate for 3 or 7 d were analyzed. Progressive differentiation was confirmed by reprobing for skeletal-muscle actin expression. Only 5 μg of total RNA from sEND.1 cells (Endo), brain (Br) and spleen (Spl) were run. (C) RT-PCR analysis of the relative levels of expression of the two CD34 isoform transcripts in undifferentiated (U) and differentiated (D) skeletal muscle cultures.
Figure 2
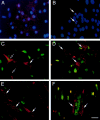
CD34 expression on a subset of muscle cells that are phenotypically segregated after induced differentiation. (A and B) Cultures of sEND.1 endothelial cells (A) and proliferating C2C12 myoblasts (B) immunostained for expression of CD34 (red) and counterstained with DAPI (blue). (Arrows) CD34+ve myoblasts. (C and D) Double immunostaining of proliferating C2C12 myoblasts for expression of CD34 (red) and either Myf5 (C, green) or MyoD (D, green). Strongly CD34-expressing cells were either negative (arrow) or faintly positive (open arrow) for Myf5 (C), but negative for MyoD (D, arrow). (E and F) Differentiated C2C12 cultures double immunostained for CD34 (red) and either Myf5 (E, green) or MyoD (F, green). CD34+ve cells (arrow) remained morphologically undifferentiated and did not express Myf5 (E) or MyoD (F). Bar, 30 μm.
Figure 6
Expression of Myf5 by CD34+ve, M-cadherin+ve satellite cells associated with isolated single fibers. (A and B) A freshly isolated single fiber from a Myf5 nlacZ/+ mouse incubated in X-Gal solution (A) and counterstained with DAPI (B). (C and D) A Myf5 nlacZ/+ mouse single fiber stained for M-cadherin (C, red) and β-Gal (C, green), counterstained with DAPI (D). (E and F) A Myf5 nlacZ/+ mouse single fiber stained for CD34 (E, green) and β-Gal (E, red), counterstained with DAPI (F). The associated satellite cells (arrows) contained β-Gal activity, in contrast to the β-Gal−ve myofiber nuclei, and expressed M-cadherin and CD34. All fibers were isolated from the EDL muscle of a 6-wk-old animal. Bar: 60 μm (A and B) and 30 μm (C–F).
Figure 3
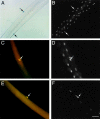
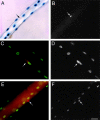
CD34 expression during skeletal muscle development. (A and B) Whole mount in situ hybridization of E 11.5 embryos for CD34 (A) and M-cadherin (B) transcripts. At E 11.5, M-cadherin was expressed at the major sites of primary myogenesis; i.e., the myotome of the somites (two representative somites are arrowed) and the limb buds (*). In contrast, CD34 transcript was present as a diffuse network throughout the embryo and in the outflow tract of the heart (arrowhead), but not in the myotome or limb buds. (C and D) Whole-mount in situ hybridization of the musculature of the shoulder region of E 16.5 embryos for CD34 (C) and M-cadherin (D). Both showed similar patterns of expression, although overlying fascia also expressed CD34 transcript (*). (E) Whole-mount in situ hybridization of the upper limb of a day 3 postnatal mouse showing CD34 expression in muscle, apparently in register with the underlying fibers. (F) A single fiber teased from day 3 postnatal muscle after hybridization with the CD34 riboprobe showing expression in a presumptive satellite cell (arrow).
Figure 4
Expression of CD34 and M-cadherin on satellite cells associated with isolated single muscle fibers. (A–C) A single EDL fiber stained for M-cadherin (A, red), CD34 (B, green), and counterstained with DAPI (C). (Arrows) Two satellite cells expressing both M-cadherin and CD34. The myonuclei, visualized by counterstaining with DAPI, did not express M-cadherin or CD34. Bar, 30 μm.
Figure 5
Expression of CD34 and MyoD on satellite cells associated with isolated single muscle fibers. (A–C) A freshly isolated EDL single fiber immunostained for CD34 (A, red), MyoD (B, green), and counterstained with DAPI (C). (Arrow) A single CD34+ve, MyoD+ve satellite cell closely associated with an underlying myonucleus. (D–F) A single EDL fiber immunostained for CD34 (D, red), MyoD (E, green), and counterstained with DAPI (F) after 48 h in culture. Two CD34+ve, MyoD+ve satellite cells (arrows) are shown migrating off the parent fiber. The CD34 staining was punctate, in contrast to the continuous surface staining observed on quiescent cells. Bar, 30 μm. (G) RT-nested PCR analysis of CD34 isoform expression in isolated single fibers during activation in vitro. Each pair of gels shows the PCR products obtained from 12 individual fibers using primers for CD34 (left) and MyoD (right). Groups of fibers were taken immediately after isolation (0 h) and after 3 or 6 h of culture in the presence of horse serum. PCR products derived from the CD34trunc and CD34full alternately spliced transcripts are arrowed T (416 bp) and F (250 bp), respectively.
Figure 9
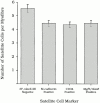
Comparison of satellite cell numbers associated with isolated EDL muscle fibers determined by counting non–3F-_nlacZ_-2E transgene-expressing nuclei, and M-cadherin+ve, CD34+ve, and Myf5+ve cells. Although the mean numbers of M-cadherin (4.5 ± 0.2, n = 114), CD34 (4.4 ± 0.2, n = 124), and Myf5-expressing cells (4.4 ± 0.3, n = 122) per EDL myofiber were not significantly different from each other (P < 0.05), the mean number of non–3F-_nlacZ_-2E transgene-expressing nuclei (5.56 ± 0.28) was significantly higher (P < 0.05). Values are expressed as population means ± SEM of the pooled data from six age-matched 3F-nlacZ_-2E transgenic mice, and from six age-matched Myf5 nlacZ/+_mice.
Figure 7
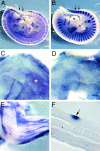
Expression of Myf5 and CD34 in satellite cells in vivo. (A) An intact EDL muscle from a 6-wk-old Myf5 nlacZ/+ mouse after incubation in X-Gal solution, showing a punctate pattern of β-Gal activity. (B–D) A transverse cryosection of the TA muscle of a 6-wk-old Myf5 nlacZ/+ mouse immunostained for β-Gal (B, D, F, and G, green) and CD34 (C–G, red) and counterstained with DAPI (D, blue). β-Gal+ve satellite cells (arrows) also expressed CD34, whereas the myonuclei (D, open arrows) were β-Gal−ve and CD34−ve. Extensive interstitial CD34 expression was observed, particularly on capillaries (C, D, and G, arrowheads). Bar: 30 μm (A–D) and 9.5 μm (E–G).
Figure 8
Expression of the 3F-_nlacZ_-2E transgene in myonuclei, but not associated satellite cells of isolated EDL single fibers. (A and B) A freshly isolated single fiber from a 3F-_nlacZ_-2E mouse after incubation in X-Gal (A), counterstained with DAPI (B). The 3F-_nlacZ_-2E transgene is expressed in all myonuclei. DAPI fluorescence is masked in β-Gal+ve nuclei, leaving non–transgene-expressing nuclei readily detectable (A and B, arrow). (C and D) A single 3F-_nlacZ_-2E muscle fiber stained for M-cadherin (C, red) and β-Gal (C, green), counterstained with DAPI (D). (E and F) A single 3F-_nlacZ_-2E muscle fiber stained for CD34 (E, red) and β-Gal (E, green), counterstained with DAPI (F). CD34+ve and M-cadherin+ve satellite cells (arrows) did not express the transgene. Bar: 30 μm (A, B, E, and F) and 19 μm (C and D).
Similar articles
- Kinetics of myoblast proliferation show that resident satellite cells are competent to fully regenerate skeletal muscle fibers.
Zammit PS, Heslop L, Hudon V, Rosenblatt JD, Tajbakhsh S, Buckingham ME, Beauchamp JR, Partridge TA. Zammit PS, et al. Exp Cell Res. 2002 Nov 15;281(1):39-49. doi: 10.1006/excr.2002.5653. Exp Cell Res. 2002. PMID: 12441128 - The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD.
Yablonka-Reuveni Z, Rudnicki MA, Rivera AJ, Primig M, Anderson JE, Natanson P. Yablonka-Reuveni Z, et al. Dev Biol. 1999 Jun 15;210(2):440-55. doi: 10.1006/dbio.1999.9284. Dev Biol. 1999. PMID: 10357902 Free PMC article. - In vivo satellite cell activation via Myf5 and MyoD in regenerating mouse skeletal muscle.
Cooper RN, Tajbakhsh S, Mouly V, Cossu G, Buckingham M, Butler-Browne GS. Cooper RN, et al. J Cell Sci. 1999 Sep;112 ( Pt 17):2895-901. doi: 10.1242/jcs.112.17.2895. J Cell Sci. 1999. PMID: 10444384 - Defining the transcriptional signature of skeletal muscle stem cells.
Yablonka-Reuveni Z, Day K, Vine A, Shefer G. Yablonka-Reuveni Z, et al. J Anim Sci. 2008 Apr;86(14 Suppl):E207-16. doi: 10.2527/jas.2007-0473. Epub 2007 Sep 18. J Anim Sci. 2008. PMID: 17878281 Free PMC article. Review. - Function of the myogenic regulatory factors Myf5, MyoD, Myogenin and MRF4 in skeletal muscle, satellite cells and regenerative myogenesis.
Zammit PS. Zammit PS. Semin Cell Dev Biol. 2017 Dec;72:19-32. doi: 10.1016/j.semcdb.2017.11.011. Epub 2017 Nov 15. Semin Cell Dev Biol. 2017. PMID: 29127046 Review.
Cited by
- Deciphering the molecular logic of WOX5 function in the root stem cell organizer.
Zhang N, Bitterli P, Oluoch P, Hermann M, Aichinger E, Groot EP, Laux T. Zhang N, et al. EMBO J. 2024 Nov 18. doi: 10.1038/s44318-024-00302-2. Online ahead of print. EMBO J. 2024. PMID: 39558109 - Mitochondrial Dynamics Drive Muscle Stem Cell Progression from Quiescence to Myogenic Differentiation.
Sommers O, Tomsine RA, Khacho M. Sommers O, et al. Cells. 2024 Oct 26;13(21):1773. doi: 10.3390/cells13211773. Cells. 2024. PMID: 39513880 Free PMC article. Review. - Genome-wide association study for growth traits with 1066 individuals in largemouth bass (Micropterus salmoides).
Han W, Qi M, Ye K, He Q, Yekefenhazi D, Xu D, Han F, Li W. Han W, et al. Front Mol Biosci. 2024 Sep 25;11:1443522. doi: 10.3389/fmolb.2024.1443522. eCollection 2024. Front Mol Biosci. 2024. PMID: 39385983 Free PMC article. - Beyond the bulk: overview and novel insights into the dynamics of muscle satellite cells during muscle regeneration.
Byun WS, Lee J, Baek JH. Byun WS, et al. Inflamm Regen. 2024 Sep 26;44(1):39. doi: 10.1186/s41232-024-00354-1. Inflamm Regen. 2024. PMID: 39327631 Free PMC article. Review. - We need to talk-how muscle stem cells communicate.
Majchrzak K, Hentschel E, Hönzke K, Geithe C, von Maltzahn J. Majchrzak K, et al. Front Cell Dev Biol. 2024 Jul 10;12:1378548. doi: 10.3389/fcell.2024.1378548. eCollection 2024. Front Cell Dev Biol. 2024. PMID: 39050890 Free PMC article. Review.
References
- Akashi K., Kondo M., Cheshier S., Shizuru J., Gandy K., Domen J., Medius R., Traver D., Weissman I.L. Lymphoid development from stem cells and the common lymphoid progenitors. Cold Spring Harbor Symp. Quant. Biol. 1999;64:1–12. - PubMed
- Baroffio A., Hamann M., Bernheim L., Bochaton-Piallat M.-L., Gabbiani G., Bader C.R. Identification of self-renewing myoblasts in the progeny of single human muscle satellite cells. Differentiation. 1996;60:47–57. - PubMed
- Baumhueter S., Dybdal N., Kyle C., Lasky L.A. Global vascular expression of murine CD34, a sialomucin-like endothelial ligand for L-selectin. Blood. 1994;84:2554–2565. - PubMed
- Bianco P., Cossu G. Uno, nessuno e centomilasearching for the identity of mesodermal progenitors. Exp. Cell Res. 1999;251:257–263. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous