DCAMKL1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization - PubMed (original) (raw)
DCAMKL1 encodes a protein kinase with homology to doublecortin that regulates microtubule polymerization
P T Lin et al. J Neurosci. 2000.
Abstract
Doublecortin (DCX) is a microtubule-associated protein required for neuronal migration to the cerebral cortex. DCAMKL1 consists of an N terminus that is 65% similar to DCX throughout the entire length of DCX, but also contains an additional 360 amino acid C-terminal domain encoding a putative Ca(2+)/calmodulin-dependent protein kinase. The homology to DCX suggested that DCAMKL1 may regulate microtubules, as well as mediate a phosphorylation-dependent signal transduction pathway. Here we show that DCAMKL1 is expressed throughout the CNS and PNS in migrating neuronal populations and overlaps in its expression with DCX and microtubules. Purified DCAMKL1 associates with microtubules and stimulates polymerization of purified tubulin and the formation of aster-like microtubule structures. Overexpressed DCAMKL1 leads to striking microtubule bundling in cell lines and cultured primary neural cells. Time-lapse imaging of cells transfected with a DCAMKL1-green fluorescent protein fusion protein shows that the microtubules associated with the protein remain dynamic. DCAMKL1 also encodes a functional kinase capable of phosphorylating myelin basic protein and itself. However, elimination of the kinase activity of DCAMKL1 has no detectable effect on its microtubule polymerization activity. Because DCAMKL1 is coexpressed with DCX, the two proteins form a potentially mutually regulatory network linking calcium signaling and microtubule dynamics.
Figures
Fig. 1.
Developmental Western and antibody specificity of DCAMKL1 antibody. A, Immunoblot analysis demonstrating specificity of α-DCAMKL1 antisera. Whole-cell extract from cultured neurons or COS7 cells transfected with an epitope-tagged expression vector encoding full-length DCAMKL1 was probed with α-DCAMKL1. α-DCAMKL1 produces a specific band at 80 kDa both in cultured neurons and as a Myc-tagged fusion protein in overexpressing COS7 cells (90 kDa). B, Developmental regulation of DCAMKL1 in various aged human occipital cortex. Blots were probed with α-DCAMKL1 and subsequently probed with α-tubulin to control for protein loading. DCAMKL1 is expressed highly during embryonic life, then rapidly downregulated, with continued expression in the adult.
Fig. 2.
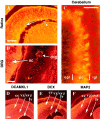
DCAMKL1 is expressed by populations of migrating neurons in the CNS and PNS. All immunostained tissues are from mouse at E14 in the sagittal plane except for the cerebellum (P8). DCAMKL1 is expressed by retinal cells predominantly in the ganglion cell layer (A), by spinal cord neurons as well as peripheral neurons in the dorsal root ganglia (B), and by cells in the internal granule layer (igl) and deeper parts of the external granule layer (egl) of the cerebellum (C). In C, sections were colabeled with α-DCAMKL1 and Calbindin. Scale bars:A, 100 μm; B, 200 μm;C, 70 μm. D–F, DCAMKL1 is expressed in similar populations of neurons as DCX, but also more widely in ventricular zones. Whereas DCX is excluded from the ventricular zone (E), DCAMKL1 immunoreactivity is present in the cortical plate (cc) as well as in the vz/svz (D). MAP2 is expressed specifically in postmitotic neurons and shows a distribution similar to DCX (F). Scale bar: D, 200 μm.cc, Cortical plate; vz, ventricular zone;lv, lateral ventricle; egl, external granule layer; pc, Purkinje cell layer;igl, internal granule layer; r, retina;l, lens; sc, spinal cord;drg, dorsal root ganglion.
Fig. 3.
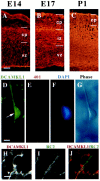
DCAMKL1 is present in migrating neurons in the cortex. A–C, Expression of DCAMKL1 in the cortex at different embryonic stages (E14, E17, and P1). DCAMKL1 is expressed widely throughout the entire cortical thickness, with less staining in the vz and intense staining in the iz, cp, and mz. Scale bars:A–C, 90 μm. vz, Ventricular zone; iz, intermediate zone; cp, cortical plate; mz, marginal zone. D–G, DCAMKL1 is expressed in bipolar cells (probably neurons) that are apposed to radial glia isolated by the cortical imprint method (Anton et al., 1999). Cortical imprint of an individual radial unit demonstrates a cell expressing DCAMKL1 (D) closely apposed to a radial glial fiber stained with Rat-401 antibody (E); F shows a DAPI nuclear counterstain, whereas G shows a matching phase-contrast image. Scale bar, 10 μm. H–J, DCAMKL1 is expressed in bipolar cells apposed to radial glia, highly suggestive of radially migrating neurons, seen in E14 cortical sections costained with anti-DCAMKL1 and RC2 antibody (Misson et al., 1988) to label radial glial cells. A radially oriented cell (H, and_red_ in J) is seen apposed to a radial glial fiber stained with RC2 (I, and_green_ in J). Scale bar, 10 μm.
Fig. 4.
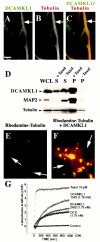
DCAMKL1 associates with microtubules and leads to microtubule polymerization. A–C, Subcellular localization of DCAMKL1 shows some localization that overlaps with microtubules. DCAMKL1 immunostaining of untreated E17 rat primary cortical neural cells (likely a neuron) demonstrates overlapping patterns of expression of DCAMKL1 with microtubules using α-tubulin antibody. DCAMKL1 and tubulin both were localized mainly to the processes of neurons. Scale bar, 10 μm. D, DCAMKL1 coprecipitates with taxol-stabilized microtubules from rat brain. Whole brain lysates from P5 newborn rat pups were cleared by centrifugation to isolate a tubulin-rich fraction and divided into two equal aliquots. To one aliquot, taxol and GTP were added and to the other aliquot, GTP alone was added, and microtubules were isolated by centrifugation. DCAMKL1 is enriched in the taxol-stabilized microtubule pellet (P, +Taxol), with smaller amounts remaining in the supernatant (S, +Taxol), whereas DCAMKL1 is not present in the pellet in the absence of taxol (P, −Taxol) and is retained in the supernatant (S, −Taxol). Reprobing a similar blot with α-pan-MAP2 indicates that DCAMKL1 distribution is similar to that of MAP2C. _WCL,_Whole-cell lysate; p, microtubule pellet;S, supernatant. E, F, Fluorescent images of rhodamine-tubulin plus phosphocellulose-purified tubulin in the absence of DCAMKL1 (E) and in the presence of 0.01 mg/ml recombinant DCAMKL1 (F) show that addition of DCAMKL1 leads to an increase in the number of microtubules (arrows) and the formation of aster-like stars.G, Turbidimetric analysis of microtubule polymerization induced by DCAMKL1. Recombinant DCAMKL1 with a C-terminal (DCAMKL1 1HIS) histidine tag, or with both C-terminal and N-terminal HIS tags (DCAMKL1 2 HIS) were incubated with PCP tubulin along with a comparable concentration of HIS-tagged DCX, or taxol as a positive control. Refracted light at 345 nm indicates light scattering because of microtubule polymerization, and shows that DCX and DCAMKL1 have comparable molar effects.
Fig. 5.
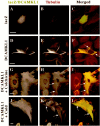
Overexpression of DCAMKL1 in cultured neural cells leads to a microtubule bundling phenotype that is resistant to depolymerization with either cold or colchicine treatment.A–C, Transfection of neural cells (in this case a likely glial cell based on morphology) with lacZ-encoding vector leads to no change in microtubule bundling and a diffuse distribution of lacZ within the cytoplasm. D–F, Transfection of cells with DCAMKL1-Myc leads to microtubule bundling (F, arrows). Scale bar, 10 μm. _G–I,_Overexpression of DCAMKL1 stabilizes microtubules to colchicine treatment (H, I, arrows), whereas surrounding untransfected cells (data not shown) or cells expressing low levels of DCAMKL1 have destabilized microtubules. _J–L,_Microtubule bundles induced by overexpression of DCAMKL1 are partially resistant to cold (0°C) treatment (K, L, arrows), whereas microtubules in neighboring cells are disrupted (data not shown). Scale bars: A, D, G, J, 10 μm.
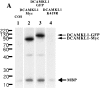
Fig. 6.
In vitro kinase activity of DCAMKL1 fusion proteins is blocked by a K419R point mutation. The various constructs of DCAMKL1 used in this study are labeled above the blot. COS7 cell extracts were transfected with either no construct (COS, 1), DCAMKL1-Myc (2), DCAMKL1-GFP (3), or the kinase-inactive K419R DCAMKL1-GFP (4) constructs. Transfection and immunoprecipitation with anti-Myc or anti-DCAMKL1 antibody was performed as described in Materials and Methods. Samples from the following assay mixtures were loaded: lane 1, nontransfected cells with MBP substrate; lane 2, DCAMKL1-Myc transfected cells with MBP; lane 3, DCAMKL1-GFP transfected cells with MBP; lane 4, K419R transfected cells with MBP. Ten micrograms of MBP were used for each reaction. Some phosphorylation of MBP is seen in untransfected COS cells (1) that is comparable with the level of phosphorylation seen in the kinase-dead DCAMKL1 construct (4). Expression of DCAMKL1 as a Myc or GFP fusion shows phosphorylation of DCAMKL1 and MBP, as well as an unidentified ≈40 kDa band that is probably a degradation product of DCAMKL1. This degradation product was not seen in other experiments with a shorter interval between reaction and loading and was more prominent with a longer postreaction interval (data not shown). All reactions were performed simultaneously and separated and transferred on the same gel and developed on the same blot and same piece of film at the same time; however, some lanes of the final gel are not illustrated for simplicity.
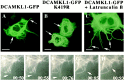
Fig. 7.
Overexpression of GFP-tagged DCAMKL1 in COS7 cells and NIH 3T3 cells induces microtubule bundling with maintained microtubule dynamics (A). Transfection of COS7 cells with DCAMKL1-GFP leads to a striking amount of microtubule bundling (arrows). B, Transfection of COS7 cells with kinase-dead DCAMKL1-GFP K419R leads to no apparent difference in degree of microtubule bundling from that of wild-type construct. C, Addition of 0.2 μ
m
Latrunculin B to COS7 cells transfected with DCAMKL1-GFP leads to the development of multiple processes containing bundled microtubules (arrows). D–H, Video frames taken from a time-lapse recording of a single NIH 3T3 cell (which show similar microtubule bundling effects to COS7 cells) showing DCAMKL1-GFP labeled microtubules and maintenance of microtubule dynamics. During this time, a single microtubule (arrow) is seen to collapse and reextend. Scale bars: A–C, 10 μm.
Similar articles
- Doublecortin-like kinase is associated with microtubules in neuronal growth cones.
Burgess HA, Reiner O. Burgess HA, et al. Mol Cell Neurosci. 2000 Nov;16(5):529-41. doi: 10.1006/mcne.2000.0891. Mol Cell Neurosci. 2000. PMID: 11083916 - DCAMKL1, a brain-specific transmembrane protein on 13q12.3 that is similar to doublecortin (DCX).
Sossey-Alaoui K, Srivastava AK. Sossey-Alaoui K, et al. Genomics. 1999 Feb 15;56(1):121-6. doi: 10.1006/geno.1998.5718. Genomics. 1999. PMID: 10036192 - The DCX-domain tandems of doublecortin and doublecortin-like kinase.
Kim MH, Cierpicki T, Derewenda U, Krowarsch D, Feng Y, Devedjiev Y, Dauter Z, Walsh CA, Otlewski J, Bushweller JH, Derewenda ZS. Kim MH, et al. Nat Struct Biol. 2003 May;10(5):324-33. doi: 10.1038/nsb918. Nat Struct Biol. 2003. PMID: 12692530 - DCX's phosphorylation by not just another kinase (JNK).
Reiner O, Gdalyahu A, Ghosh I, Levy T, Sapoznik S, Nir R, Sapir T. Reiner O, et al. Cell Cycle. 2004 Jun;3(6):747-51. Epub 2004 Jun 6. Cell Cycle. 2004. PMID: 15118415 Review. - Doublecortin functions at the extremities of growing neuronal processes.
Friocourt G, Koulakoff A, Chafey P, Boucher D, Fauchereau F, Chelly J, Francis F. Friocourt G, et al. Cereb Cortex. 2003 Jun;13(6):620-6. doi: 10.1093/cercor/13.6.620. Cereb Cortex. 2003. PMID: 12764037 Review.
Cited by
- Doublecortin-like kinase is required for cnidocyte development in Nematostella vectensis.
Kraus JEM, Busengdal H, Kraus Y, Hausen H, Rentzsch F. Kraus JEM, et al. Neural Dev. 2024 Jun 22;19(1):11. doi: 10.1186/s13064-024-00188-0. Neural Dev. 2024. PMID: 38909268 Free PMC article. - A novel antibody against cancer stem cell biomarker, DCLK1-S, is potentially useful for assessing colon cancer risk after screening colonoscopy.
Sarkar S, Popov VL, O'Connell MR, Stevenson HL, Lee BS, Obeid RA, Luthra GK, Singh P. Sarkar S, et al. Lab Invest. 2017 Oct;97(10):1245-1261. doi: 10.1038/labinvest.2017.40. Epub 2017 Apr 17. Lab Invest. 2017. PMID: 28414327 Free PMC article. - DCLK1, a promising colorectal cancer stem cell marker, regulates tumor progression and invasion through miR-137 and miR-15a dependent manner.
Razi S, Sadeghi A, Asadi-Lari Z, Tam KJ, Kalantari E, Madjd Z. Razi S, et al. Clin Exp Med. 2021 Feb;21(1):139-147. doi: 10.1007/s10238-020-00665-w. Epub 2020 Sep 23. Clin Exp Med. 2021. PMID: 32965580 - Doublecotin-Like Kinase 1 Increases Chemoresistance of Colorectal Cancer Cells through the Anti-Apoptosis Pathway.
Li L, Jones K, Mei H. Li L, et al. J Stem Cell Res Ther. 2019;9(3):447. doi: 10.4172/2157-7633.1000447. Epub 2019 May 3. J Stem Cell Res Ther. 2019. PMID: 31372308 Free PMC article. - DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer.
Bailey JM, Alsina J, Rasheed ZA, McAllister FM, Fu YY, Plentz R, Zhang H, Pasricha PJ, Bardeesy N, Matsui W, Maitra A, Leach SD. Bailey JM, et al. Gastroenterology. 2014 Jan;146(1):245-56. doi: 10.1053/j.gastro.2013.09.050. Epub 2013 Oct 2. Gastroenterology. 2014. PMID: 24096005 Free PMC article.
References
- Anton ES, Kreidberg JA, Rakic P. Distinct functions of alpha3 and alpha(v) integrin receptors in neuronal migration and laminar organization of the cerebral cortex. Neuron. 1999;22:277–289. - PubMed
- Avila J, Dominguez J, Diaz-Nido J. Regulation of microtubule dynamics by microtubule-associated protein expression and phosphorylation during neuronal development. Int J Dev Biol. 1994;38:13–25. - PubMed
- Baas PW. Microtubules and neuronal polarity: lessons from mitosis. Neuron. 1999;22:23–31. - PubMed
- Benezra R, Davis RL, Lockshon D, Turner DL, Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990;61:49–59. - PubMed
- Berg MJ, Schifitto G, Powers JM, Martinez-Capolino C, Fong CT, Myers GJ, Epstein LG, Walsh CA. X-linked female band heterotopia-male lissencephaly syndrome. Neurology. 1998;50:1143–1146. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- K12 NS001701/NS/NINDS NIH HHS/United States
- P01 39404/PHS HHS/United States
- R01 NS041537/NS/NINDS NIH HHS/United States
- R01 NS38097/NS/NINDS NIH HHS/United States
- 5K12NS01701/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous