Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity - PubMed (original) (raw)
Fine-tuning an auditory synapse for speed and fidelity: developmental changes in presynaptic waveform, EPSC kinetics, and synaptic plasticity
H Taschenberger et al. J Neurosci. 2000.
Abstract
Fast, precise, and sustained synaptic transmission at high frequency is thought to be crucial for the task of sound localization in the auditory brainstem. However, recordings from the calyx of Held synapse have revealed severe frequency-dependent synaptic depression, which tends to degrade the exact timing of postsynaptic spikes. Here we investigate the functional changes occurring throughout the critical period of synapse refinement from immature calyx terminal [postnatal day 5 (P5)] to after the onset of hearing (P12-P14). Surprisingly, for recordings near physiological temperature (35 degrees C), we find that P14 synapses are already able to follow extremely high input rates of up to 800 Hz. This ability stems in part from a remarkable shortening of presynaptic action potentials, which may lead to a lowering of release probability and decrease in synaptic delays during development. In addition, AMPA receptor-mediated EPSCs as well as quantal synaptic currents acquired progressively faster kinetics, although their mean amplitudes did not change significantly. NMDA receptor-mediated EPSCs, however, diminished with age, as indicated by a 50% reduction in mean amplitude and faster decay kinetics. Finally, the degree of synaptic depression was greatly attenuated with age, presumably because of a 2.5-fold or larger increase in the releasable pool of vesicles, which together with a decreasing release probability produces a fairly constant EPSC amplitude. This finely tuned orchestra of developmental changes thus simultaneously promotes speed while preventing premature vesicle pool depletion during prolonged bouts of firing. A few critical days in postnatal development can thus have a large impact on synaptic function.
Figures
Fig. 1.
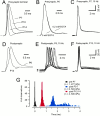
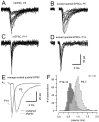
Developmental changes in waveform and timing of presynaptic and postsynaptic APs at the calyx of Held synapse.A, Current-clamp whole-cell recordings of presynaptic APs in brainstem slices at P7, P10, and P14. APs were evoked by afferent fiber stimulation. Note the faster kinetics and shorter duration of the APs and the presence of a fast afterhyperpolarization in older calyxes. B, Presynaptic AP waveform for two different P6 calyxes. One calyx was dialyzed with 0.2 m
m
EGTA, whereas the other was dialyzed with 5 m
m
EGTA.C, Consecutive responses to a train of afferent stimuli (10 Hz) recorded in a presynaptic terminal at P7 (same neuron as in_A_, first 10 responses). D, Comparison of AP waveforms in postsynaptic MNTB neurons in P6 and P13 slices.E, F, Consecutive responses to afferent stimulation at 10 Hz (same neurons as in D, first 10 responses).G, Latency distribution of APs elicited at 10 Hz. Pooled data from P5–P7 (n = 6) and P12–P14 (n = 4) slices. Latency was calculated as the time from negative peak of the prepotential to the peak of the postsynaptic APs. During development, the latency between presynaptic and postsynaptic APs decreased and the jitter in AP timing was reduced. Mean latency was 2.09 ± 0.24 and 0.64 ± 0.03 msec for P5–P7 and P12–P14 slices, respectively. Latency distribution of the presynaptic APs is shown for comparison (same neuron as in_C_). K-gluconate-filled electrodes were used and experiments were done at room temperature. The resting membrane potential (_V_r) was −80 and −70 mV for presynaptic and postsynaptic neurons, respectively. Stimulus artifacts were blanked for clarity.
Fig. 2.
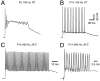
The capacity for high-frequency transmission is fully established by the end of the second postnatal week. A, B, Postsynaptic APs in response to trains of 15 stimuli (100 Hz) recorded in P5 (A) and P14 (B) brainstem slices at room temperature. At P5, usually only the first two to three EPSPs were suprathreshold and generated APs in the postsynaptic neuron. Note the strong AP adaptation and large plateau depolarization at P5, which are both absent at P14.C, D, Current-clamp recordings at near physiological temperature (35°C). In P12–P14 slices, reliable synaptic transmission was generally possible at frequencies up to 600 Hz under these conditions (50 stimuli; C). Occasionally, postsynaptic neurons were able to respond to afferent stimulation up to 800 Hz without failures of AP generation (15 stimuli;D). K-gluconate-filled electrodes._V_r was approximately −70 mV. Notice that for the 800 Hz frequency the exact timing of the APs late in the train becomes less precise, as evidenced by the stimulus artifact falling progressively further into the down stroke phase of the preceding spike (D).
Fig. 3.
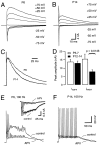
During postnatal development, NMDA receptor-mediated EPSCs decreased in amplitude and acquired faster kinetics. A, B, Families of evoked EPSCs were recorded at various holding potentials (V_h, as indicated) in P6 (A) and P14 (B) brainstem slices. At P14, amplitudes of_I_NMDA were largely reduced, whereas_I_AMPA remained approximately constant throughout development. C, Same neurons as depicted in_A and B. To facilitate comparison, EPSCs evoked at _V_h = +50 mV are shown superimposed. Current responses were normalized and aligned at their peak amplitudes of _I_NMDA. D, Summary data of five and eight different cells for P5–P7 and P12–P14, respectively. Mean amplitudes of peak _I_AMPAwere −13.76 ± 1.36 and −12.96 ± 1.25 nA (_V_h = −75 mV), and mean amplitudes of peak _I_NMDA were 15.4 ± 2.92 and 7.8 ± 1.06 nA (_V_h = +50 mV) for P5–P7 and P12–P14, respectively. E, F, Current-clamp recordings of postsynaptic APs evoked by afferent fiber stimuli (100 Hz) under control conditions and during application of 50 μ
md,l
-APV (_V_r = −70 mV). E, At P6 (n = 6 cells), APV strongly reduced the large plateau depolarization but had no effect on the number of APs generated during the stimulus train. Inset, Voltage-clamp recordings (_V_h = −30 mV) from the same cell; AMPA EPSCs truncated for clarity. F, At P14, 50 μ
md,l
-APV had little effect on postsynaptic responses. CsCl and K-gluconate-based internal solutions were used in the experiments illustrated in A–D and _E_and F, respectively.
Fig. 4.
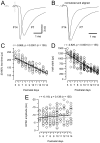
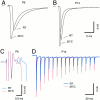
Developmental changes in synaptic current kinetics for AMPA receptor-mediated EPSCs. A, Sample EPSCs recorded in P7 and P14 brainstem slices (V_h = −70 mV). Current responses were aligned at the negative peak of the prepotential (dotted line). Note the shorter synaptic delay and faster time course at P14. B, Same responses as depicted in_A. To facilitate comparison, amplitudes were normalized, and EPSCs were aligned at their onsets. C–E, Pooled data for EPSC rise times (C), half widths (D), and peak amplitudes (E) from a total of >130 cells plotted versus postnatal age. Solid and _dotted lines_indicate linear regression and 99% confidence limits, respectively. Regression coefficients and number of cells as indicated at each panel. Both EPSC rise times and half widths decreased significantly (p < 0.0001) during development. In contrast, only little increase in EPSC peak amplitudes was observed (_p_ > 0.05).
Fig. 5.
Asynchronous transmitter release does not account for slower EPSC waveforms in P5–P7 slices. A, C, Spontaneous miniature EPSCs recorded at P5 (A) and P14 (C) are superimposed and aligned at their peak amplitude. B, D, Evoked quantal EPSCs recorded at greatly reduced probability of release (50 μ
m
CdCl2 included in the bath solution) at P5 (B) and P14 (D). Occasionally occurring EPSCs with amplitudes >150 pA were discarded. The events in A–D are from four different cells, and 15 sample traces are shown superimposed. mEPSCs were collected under the same recording conditions (with 50 μ
m
CdCl2) from 10 to 200 msec after the stimulus. Note the difference in synaptic delays and EPSC kinetics in _B_and D. Stimulus frequency for evoked quantal events was 1–2 Hz. E, Average waveforms of quantal EPSCs were obtained either with (dotted lines) or without (solid lines) previous aligning of individual EPSCs at their 50% rise points. 170 and 125 responses were averaged for P5 and P14, respectively. At both ages, aligned EPSC gave rise to slightly faster kinetics of the averaged EPSC waveform. 20–80% rise times were 221 versus 310 μsec (P5) and 128 versus 176 μsec (P14) for the aligned and unaligned EPSCs, respectively. Half widths were 1406 versus 1477 μsec (P5) and 441 versus 541 μsec (P14) for waveforms obtained from aligned and unaligned EPSC, respectively. F, Latency distribution of quantal EPSC. Latency was calculated as the time difference between negative peak of the prepotential and 20% rise point of the EPSCs. Pooled data obtained from four different cells in each age group.
Fig. 6.
Frequency-dependent short-term depression at the calyx of Held synapse during development. A–D, EPSC trains evoked by afferent stimulation at 10 Hz (25 stimuli;A, C) and 100 Hz (15 stimuli;B, D). Data from two different cells (A, B, P6; C,D, P14) with similar peak amplitudes of the initial EPSCs are shown. V_h = −70 mV.E, F, Same neuron as depicted in C and_D. Stimulation frequency was 300 Hz (E) and 500 Hz (F). For_A–F_, mean amplitudes of depressed EPSCs (last five EPSCs during a train) amounted to 1.91 nA (10 Hz), 0.32 nA (100 Hz), and 6.45 nA (10 Hz), 5.41 nA (100 Hz), 2.98 nA (300 Hz), and 1.95 nA (500 Hz) for P6 and P14, respectively.
Fig. 7.
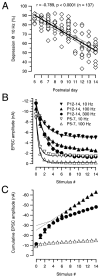
Synaptic depression and vesicle pool size during postnatal development. A, Synaptic depression at 10 Hz. The ratio between the initial EPSC over the mean steady-state depressed EPSC (last five stimuli) after 25 stimuli was calculated for each cell and expressed as a percentage. Pooled data for a total of 115 cells plotted versus postnatal age. Solid and dotted lines indicate linear regression and 99% confidence limits, respectively. B, Average data of synaptic depression for various stimulation frequencies in P5–P7 (open symbols) and P12–P14 (filled symbols) slices. Three frequencies (10, 100, and 300 Hz; n = 25 cells) were tested for each age group. For P5–P7, depression obtained with 100 and 300 Hz was similar, and therefore only the 100 Hz data points were included.Solid lines represent single exponential fits to the data points (τ values for 10 and 100 Hz were 69.2 and 6.1 msec for P5–P7 vs 233.3 and 23.2 msec for P12–P14, respectively). Note that a 30 times higher stimulation frequency was necessary to obtain a similar amount of depression in P12–P14 slices as seen with 10 Hz at P5–P7.C, Cumulative EPSC amplitudes obtained by adding EPSC amplitudes during the 100 and 300 Hz trains (same symbols as in_B_) to estimate change in vesicle pool sizes.Dotted lines represent linear fits to the last five EPSC amplitudes during the train back-extrapolated to 0 to estimate the cumulative EPSC amplitudes before steady-state depression. At P12–P14, the estimated pool size was 2.5–3× larger than P5–P7.
Fig. 8.
Temperature dependence of synaptic delay, EPSC time course, and synaptic depression. A, B, Comparison of EPSC waveforms at RT and 35°C for P6 (A) and P14 (B) brainstem slices. The 20–80% rise times were 220 and 136 msec (P6) and 122 and 95 msec (P14) for RT and 35°C, respectively.C, Temperature-dependent shift of prepotential and EPSC onset in another P6 neuron shown at a faster time scale.D, Comparison of EPSCs evoked by high-frequency afferent stimulation (100 Hz) at room temperature (blue trace) and 35°C (red trace). Same neuron as shown in_B_. In this neuron, synaptic depression was reduced from 77% at room temperature to 65% at 35°C. In these examples, the temperature was raised from RT (21–23°C) to 35°C in <5 min, and all effects were fully reversible.
Similar articles
- Presynaptic Diversity Revealed by Ca2+-Permeable AMPA Receptors at the Calyx of Held Synapse.
Lujan B, Dagostin A, von Gersdorff H. Lujan B, et al. J Neurosci. 2019 Apr 17;39(16):2981-2994. doi: 10.1523/JNEUROSCI.2565-18.2019. Epub 2019 Jan 24. J Neurosci. 2019. PMID: 30679394 Free PMC article. - High-fidelity transmission acquired via a developmental decrease in NMDA receptor expression at an auditory synapse.
Futai K, Okada M, Matsuyama K, Takahashi T. Futai K, et al. J Neurosci. 2001 May 15;21(10):3342-9. doi: 10.1523/JNEUROSCI.21-10-03342.2001. J Neurosci. 2001. PMID: 11331363 Free PMC article. - Physiological temperatures reduce the rate of vesicle pool depletion and short-term depression via an acceleration of vesicle recruitment.
Kushmerick C, Renden R, von Gersdorff H. Kushmerick C, et al. J Neurosci. 2006 Feb 1;26(5):1366-77. doi: 10.1523/JNEUROSCI.3889-05.2006. J Neurosci. 2006. PMID: 16452660 Free PMC article. - Vesicle pools and short-term synaptic depression: lessons from a large synapse.
Schneggenburger R, Sakaba T, Neher E. Schneggenburger R, et al. Trends Neurosci. 2002 Apr;25(4):206-12. doi: 10.1016/s0166-2236(02)02139-2. Trends Neurosci. 2002. PMID: 11998689 Review. - The calyx of Held synapse: from model synapse to auditory relay.
Borst JG, Soria van Hoeve J. Borst JG, et al. Annu Rev Physiol. 2012;74:199-224. doi: 10.1146/annurev-physiol-020911-153236. Epub 2011 Oct 24. Annu Rev Physiol. 2012. PMID: 22035348 Review.
Cited by
- Strength and precision of neurotransmission at mammalian presynaptic terminals.
Takahashi T. Takahashi T. Proc Jpn Acad Ser B Phys Biol Sci. 2015;91(7):305-20. doi: 10.2183/pjab.91.305. Proc Jpn Acad Ser B Phys Biol Sci. 2015. PMID: 26194855 Free PMC article. Review. - Presynaptic nanodomains: a tale of two synapses.
Wang LY, Augustine GJ. Wang LY, et al. Front Cell Neurosci. 2015 Jan 26;8:455. doi: 10.3389/fncel.2014.00455. eCollection 2014. Front Cell Neurosci. 2015. PMID: 25674049 Free PMC article. Review. - The quantal component of synaptic transmission from sensory hair cells to the vestibular calyx.
Highstein SM, Mann MA, Holstein GR, Rabbitt RD. Highstein SM, et al. J Neurophysiol. 2015 Jun 1;113(10):3827-35. doi: 10.1152/jn.00055.2015. Epub 2015 Apr 15. J Neurophysiol. 2015. PMID: 25878150 Free PMC article. - Characterization of Developmental Changes in Spontaneous Electrical Activity of Medial Superior Olivary Neurons Before Hearing Onset With a Combination of Injectable and Volatile Anesthesia.
Di Guilmi MN, Rodríguez-Contreras A. Di Guilmi MN, et al. Front Neurosci. 2021 Apr 15;15:654479. doi: 10.3389/fnins.2021.654479. eCollection 2021. Front Neurosci. 2021. PMID: 33935637 Free PMC article. - The relationship between agonist potency and AMPA receptor kinetics.
Zhang W, Robert A, Vogensen SB, Howe JR. Zhang W, et al. Biophys J. 2006 Aug 15;91(4):1336-46. doi: 10.1529/biophysj.106.084426. Epub 2006 May 26. Biophys J. 2006. PMID: 16731549 Free PMC article.
References
- Bellingham MC, Walmsley B. A novel presynaptic inhibitory mechanism underlies paired pulse depression at a fast central synapse. Neuron. 1999;23:159–170. - PubMed
- Blatchley BJ, Cooper WA, Coleman JR. Development of auditory brainstem response to tone pip stimuli in the rat. Dev Brain Res. 1987;32:75–84. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous