Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system - PubMed (original) (raw)
Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system
C Cirelli et al. J Neurosci. 2000.
Abstract
Behavioral studies indicate that the ability to acquire long-term memories is severely impaired during sleep. It is unclear, however, why the highly synchronous discharge of neurons during sleep should not be followed by the induction of enduring plastic changes. Here we show that the expression of phosphorylated CRE-binding protein, Arc, and BDNF, three genes whose induction is often associated with synaptic plasticity, is high during waking and low during sleep. We also show that the induction of these genes during waking depends on the activity of the noradrenergic system, which is high in waking and low in sleep. These molecular results complement behavioral evidence and provide a mechanism for the impairment of long-term memory acquisition during sleep.
Figures
Fig. 1.
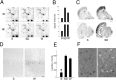
CREB phosphorylation in the cerebral cortex after sleep and waking. A, The experimental conditions chosen to distinguish changes associated with sleep (S), waking (W), circadian factors, and the sleep-deprivation procedure (SD). A 12 hr light/dark cycle is indicated by the horizontal bar. Three hours (3h) and 8 hr (8h) of S,SD, and W are shown.B, Anti-P-CREB staining in coronal sections of parietal cortex (layers II–VI) from a rat that slept for 3 hr (S) and a rat that was sleep-deprived for 3 hr (SD). Scale bar, 100 μm. C, Mean number (±SEM) of P-CREB-immunoreactive neurons in entorhinal (ent), parietal (par), temporal (te), and occipital (occ) cortex after 3 or 8 hr of S (n = 4/group), 3 or 8 hr of SD (n = 4/group), and 3 or 8 hr of spontaneous W (n = 3 after 3 hr; n = 2 after 8 hr). The sampled area was 500 μm wide across all cortical layers. The number of P-CREB-immunoreactive neurons was significantly higher in_SD_ and W with respect to S(Mann–Whitney U test, *p < 0.01). No differences were found between SD and_W_. For each condition, there were no differences between data from the 3 and 8 hr groups, which were therefore pooled.
Fig. 2.
Arc expression in the cerebral cortex after sleep and waking. A, cDNA microarrays showing cortical Arc mRNA levels (arrowheads) after 3 hr (3h) or 8 hr (8h) of sleep (S; n = 7), sleep deprivation (SD; n = 7), and waking (W; n = 6).B, Densitometric analysis performed by scanning the microarrays with a PhosphorImager. The y_-axis values refer to signal intensity (arbitrary units). C,In situ hybridization for Arc mRNA in brain sections of a representative rat killed after 8 hr of_S and of a rat killed at the same circadian time after 8 hr of SD. Scale bar, 1.5 mm. D, Arc levels measured with immunocytochemistry in the parietal cortex of rats after 3 hr of S and 3 hr of W. Scale bar, 100 μm. E, Mean number (±SEM) of Arc-immunoreactive neurons in parietal cortex after 3 hr of_S_ (n = 8), SD(n = 8), and W(n = 5). The sampled area was a 500-μm-wide cortical column spanning all layers (Mann–Whitney _U_test, *p < 0.01). F, Double labeling in the parietal cortex of a rat that was sleep deprived for 3 hr. Arc immunoreactivity (black cells;left) does not colocalize with parvalbumin immunoreactivity (white cells; right). Scale bar, 50 μm.
Fig. 3.
Arc expression in the parietal cortex as a function of the duration of waking. A, B, Arc levels measured with immunocytochemistry in cortical layers V (A) and VI (B) after 1 hr (1h), 3 hr (3h), and 9 hr (9h) of sleep deprivation (SD). Scale bar, 200 μm.
Fig. 4.
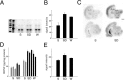
BDNF expression in the cerebral cortex after sleep and waking. A, RPA showing cortical_BDNF_ mRNA levels after 8 hr of sleep (S;n = 7), sleep deprivation (SD;n = 7), and waking (W;n = 6). A β -actin_antisense riboprobe was used to normalize the amount of sample RNA.Lane 1 (from left), Molecular weight markers. Lane 2, BDNF and_β -actin riboprobes hybridized with 10 μg of yeast RNA, incubated without RNase mixture. Most of the signal is the full-length BDNF (arrow) and_β_ -actin (arrowhead) probes. Lanes 3–8, BDNF and_β_ -actin probes hybridized under conditions of excess probe with 2 μg of pooled RNA. S,lanes 3 and 4; SD, _lanes 5_and 6; W, lanes 7 and 8. The protected fragments are 407 bp for BDNF and 250 bp for β -actin. B, Densitometric analysis performed by scanning the RPA gel with a PhosphorImager. The _y_-axis values refer to signal intensity (arbitrary units). Relative to S,BDNF mRNA levels were higher in both _SD_and W rats (t test, _p_= 0.021 and 0.028, respectively). C, In situ hybridization for BDNF mRNA in brain sections of representative rats killed after 8 hr of S_and 8 hr of SD. Scale bar, 1.5 mm. D, BDNF levels (picograms per milligram of protein) measured with ELISA in the cerebral cortex of rats after 8 hr of S,SD, and W. Each column_represents an individual rat (mean of 3 measurements ± SEM). Animals were divided into two groups on the basis of their age: 8–9 weeks (left) and 15–16 weeks (right). BDNF levels for all rats were measured simultaneously on the same assay. BDNF levels were higher in_SD and W rats than in S_rats (F = 21.11; p < 0.01).E, Densitometric analysis showing changes in_TrkB mRNA levels, as measured by RPA, after 8 hr of_S (n = 7), SD(n = 7), and W(n = 6). The _y_-axis values refer to signal intensity (arbitrary units). Relative to S, cortical TrkB mRNA levels were significantly higher after 8 hr of SD (t test,p = 0.032) but not after 8 hr of W(t test, p = 0.058).
Fig. 5.
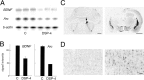
BDNF and Arc_expression in the cerebral cortex after lesion of the noradrenergic system of the locus coeruleus. A, RPA showing cortical_BDNF and Arc mRNA levels after 8 hr of sleep deprivation in control rats who received a saline injection (C; n = 4; _lanes 1–3_from left) and in rats treated with DSP-4 to destroy the noradrenergic innervation of the cerebral cortex (n_= 4; lanes 4–6). A_β -actin antisense riboprobe was used to normalize the amount of sample RNA. B, Densitometric analysis performed by scanning the RPA gel with a PhosphorImager. The_y_-axis values refer to signal intensity (arbitrary units). BDNF and Arc mRNA levels were higher in C than in DSP-4 rats (t test,p = 0.030 and 0.00, respectively). C, Left, Coronal brainstem section of a representative rat in which the left locus coeruleus was destroyed by a local injection of 6-OHDA (the arrow indicates the right locus coeruleus). Tyrosine hydroxylase immunostaining, used to identify noradrenergic neurons and fibers in the locus coeruleus, is abolished on the side of the lesion, whereas it is intact on the other side. Scale bar, 1.2 mm.Right, A rostral coronal brain section immunostained against tyrosine hydroxylase from the same animal showing the almost complete depletion of noradrenergic fibers in the left cerebral cortex and hippocampus 2 weeks after the lesion. The noradrenergic innervation on the right side of the brain is intact. Scale bar, 2 mm. D, Arc immunostaining in a coronal section of parietal cortex adjacent to that shown in C. Arc expression is high on the intact side (right) after 3 hr of sleep deprivation, but it is as low as in sleep on the side where the noradrenergic innervation had been destroyed (left). Scale bar, 100 μm.
Similar articles
- Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats.
Guzman-Marin R, Ying Z, Suntsova N, Methippara M, Bashir T, Szymusiak R, Gomez-Pinilla F, McGinty D. Guzman-Marin R, et al. J Physiol. 2006 Sep 15;575(Pt 3):807-19. doi: 10.1113/jphysiol.2006.115287. Epub 2006 Jul 6. J Physiol. 2006. PMID: 16825295 Free PMC article. - Neuronal gene expression in the waking state: a role for the locus coeruleus.
Cirelli C, Pompeiano M, Tononi G. Cirelli C, et al. Science. 1996 Nov 15;274(5290):1211-5. doi: 10.1126/science.274.5290.1211. Science. 1996. PMID: 8895474 - On the functional significance of c-fos induction during the sleep-waking cycle.
Cirelli C, Tononi G. Cirelli C, et al. Sleep. 2000 Jun 15;23(4):453-69. Sleep. 2000. PMID: 10875553 Review. - A molecular window on sleep: changes in gene expression between sleep and wakefulness.
Cirelli C. Cirelli C. Neuroscientist. 2005 Feb;11(1):63-74. doi: 10.1177/1073858404270900. Neuroscientist. 2005. PMID: 15632279 Review.
Cited by
- Acute Exposure to Microcystin-Producing Cyanobacterium Microcystis aeruginosa Alters Adult Zebrafish (Danio rerio) Swimming Performance Parameters.
Kist LW, Piato AL, da Rosa JG, Koakoski G, Barcellos LJ, Yunes JS, Bonan CD, Bogo MR. Kist LW, et al. J Toxicol. 2011;2011:280304. doi: 10.1155/2011/280304. Epub 2011 Dec 28. J Toxicol. 2011. PMID: 22253623 Free PMC article. - A neuromorphic architecture for object recognition and motion anticipation using burst-STDP.
Nere A, Olcese U, Balduzzi D, Tononi G. Nere A, et al. PLoS One. 2012;7(5):e36958. doi: 10.1371/journal.pone.0036958. Epub 2012 May 15. PLoS One. 2012. PMID: 22615855 Free PMC article. - Suppression of hippocampal plasticity-related gene expression by sleep deprivation in rats.
Guzman-Marin R, Ying Z, Suntsova N, Methippara M, Bashir T, Szymusiak R, Gomez-Pinilla F, McGinty D. Guzman-Marin R, et al. J Physiol. 2006 Sep 15;575(Pt 3):807-19. doi: 10.1113/jphysiol.2006.115287. Epub 2006 Jul 6. J Physiol. 2006. PMID: 16825295 Free PMC article. - Sleep as a Therapeutic Target in the Aging Brain.
Bah TM, Goodman J, Iliff JJ. Bah TM, et al. Neurotherapeutics. 2019 Jul;16(3):554-568. doi: 10.1007/s13311-019-00769-6. Neurotherapeutics. 2019. PMID: 31376067 Free PMC article. Review. - Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish.
Egan RJ, Bergner CL, Hart PC, Cachat JM, Canavello PR, Elegante MF, Elkhayat SI, Bartels BK, Tien AK, Tien DH, Mohnot S, Beeson E, Glasgow E, Amri H, Zukowska Z, Kalueff AV. Egan RJ, et al. Behav Brain Res. 2009 Dec 14;205(1):38-44. doi: 10.1016/j.bbr.2009.06.022. Epub 2009 Jun 18. Behav Brain Res. 2009. PMID: 19540270 Free PMC article.
References
- Berchtold NC, Oliff HS, Isackson P, Cotman CW. Hippocampal BDNF mRNA shows a diurnal regulation, primarily in the exon III transcript. Mol Brain Res. 1999;71:11–22. - PubMed
- Bloom FE. Fine structural changes in rat brain after intracisternal injection of 6-hydroxydopamine. In: Malmfors T, Thoenen H, editors. 6-Hydroxydopamine and catecholamine neurons. North-Holland; Amsterdam: 1971. pp. 135–150.
- Bova R, Micheli MR, Qualadrucci P, Grassi-Zucconi G. BDNF and trkB mRNAs oscillate in rat brain during the light-dark cycle. Mol Brain Res. 1998;57:321–324. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources