Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid X receptor alpha mediated by a tamoxifen-inducible chimeric Cre recombinase (Cre-ERT2) in adipocytes - PubMed (original) (raw)
Impaired adipogenesis and lipolysis in the mouse upon selective ablation of the retinoid X receptor alpha mediated by a tamoxifen-inducible chimeric Cre recombinase (Cre-ERT2) in adipocytes
T Imai et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Retinoid X receptor alpha (RXRalpha) is involved in multiple signaling pathways, as a heterodimeric partner of several nuclear receptors. To investigate its function in energy homeostasis, we have selectively ablated the RXRalpha gene in adipocytes of 4-week-old transgenic mice by using the tamoxifen-inducible Cre-ERT2 recombination system. Mice lacking RXRalpha in adipocytes were resistant to dietary and chemically induced obesity and impaired in fasting-induced lipolysis. Our results also indicate that RXRalpha is involved in adipocyte differentiation. Thus, our data demonstrate the feasibility of adipocyte-selective temporally controlled gene engineering and reveal a central role of RXRalpha in adipogenesis, probably as a heterodimeric partner for peroxisome proliferator-activated receptor gamma.
Figures
Figure 1
Characterization of aP2-Cre-ERT2 transgenic mice. (A) Cre-ERT2 mRNA is selectively expressed in adipose tissue of aP2-Cre-ERT2 transgenic mice. Cre-ERT2 expression was analyzed by RT-PCR on RNA extracted from WAT of a 3-month-old WT mouse and from the indicated tissues of a 3-month-old aP2-Cre-ERT2(tg/0) transgenic mouse. The PCR products corresponding to Cre-ERT2 and HPRT mRNA are indicated. (B) After Tam treatment, aP2-Cre-ERT2 transgenic mice efficiently excise a floxed EGFP cassette in adipocytes. Cryosections of WAT isolated from a 3-month-old Tam-treated aP2-Cre-ERT2(tg/0) mouse (a), vehicle-treated aP2-Cre-ERT2(tg/0)/aP2-L-EGFP-L(tg/0) bigenic mouse (b), and Tam-treated aP2-Cre-ERT2(tg/0)/aP2-L-EGFP-L(tg/0) bigenic mouse (c), 30 days after the injection, were analyzed by confocal microscopy. The blue color corresponds to 4′,6-diamidino-2-phenylindole dihydrochloride-stained nuclei, and the green color corresponds to GFP fluorescence. (Scale bar, 20 μm.) (C) Cre-ERT2 mice selectively excise floxed DNA in adipocytes after Tam treatment. Cre-ERT2-mediated DNA excision was determined by Southern blot analysis, performed on genomic DNA isolated from organs of 6-month-old aP2-Cre-ERT2(tg/0)/RXRα+/af2(I) bigenic mice, 7 days after the last injection of vehicle (lanes 2–12) or Tam (lanes 13–23) or from RXRα+/af2(II) mouse tail (lane 1). The position of the WT (+), floxed [af2(I)], and recombined [af2(II)] RXRα alleles are indicated. BAT, brown adipose tissue.
Figure 2
Adipocyte-selective RXRα ablation in mice. (A) Efficiency of RXRα inactivation in adipocytes. Cre-ERT2-mediated RXRα disruption was analyzed by Southern blotting DNA extracted from WAT (T) isolated from 4-month-old aP2-Cre-ERT2(tg/0)/RXRαL2/− mice, 3 months after vehicle (−) and Tam (+) treatments, and from the supernatant (S) and pellet (P) fractions after centrifugation of collagenase-treated WAT. Positions of RXRα L2, L−, and (−) alleles are indicated. (B) Expression of nuclear receptors, PPARγ target genes, and Pref-1 in RXRα-deficient adipose tissue. RXRβ, RXRγ, PPARγ, aP2, and LPL mRNA levels were analyzed by Northern blotting RNA isolated from WAT of 6-month-old CT (lanes 1, 3, and 5) and RXRαadL−/− (KO, lanes 2, 4, and 6) mice, under RD (lanes 1 and 2), after MSG treatment (lanes 3 and 4) and under HFD (lanes 5 and 6). 36B4 was used as an internal control. Pref-1 expression was analyzed by RT-PCR performed on the same RNA samples, and HPRT was used as an internal control. (C) Altered RXRγ and aP2 expression in the adipose tissue of RXRαadL−/− mice 2 weeks after Tam injection. RXRγ and aP2 mRNA levels were analyzed by Northern blotting RNA isolated from adipose tissue of two 6-week-old CT (lanes 1 and 2) and RXRαadL−/− (KO, lanes 3 and 4) mice, 2 weeks after Tam treatment. 36B4 was used as an internal control.
Figure 3
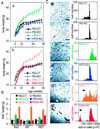
RXRαadL−/− mice are resistant to obesity and are impaired in preadipocyte differentiation. (A) RXRαadL−/− mice are resistant to HFD- and MSG-induced obesity. Total body weight of CT and RXRαadL−/− (KO) mice under RD (a and b), under HFD (a) and after MSG treatment (b) was measured weekly. The number of animals monitored in each group was 10–15 males. Values are expressed as the mean ± SEM. (B) Reduced adipose tissue weight in RXRαadL−/− mice under HFD or after MSG treatment compared with CT mice. Inguinal (ING), interscapular (INT), and retroperitoneal (RET) WAT weight was determined in 6-month-old CT and RXRαadL−/− (KO) mice, fed with RD or HFD and after MSG treatment. Each group was composed of 5–7 males. Values are expressed as the mean ± SEM. (C) Impaired adipogenesis in RXRαadL−/− mice. Cryosections of s.c. inguinal WAT from 6-month-old CT (a, c, and_e_) and RXRαadL−/− (b,d, and f) mice, under RD (a and b), HFD (c and_d_), and after MSG treatment (e and_f_) were stained with hematoxylin and eosin. (Scale bar, 160 μm.) The areas of at least 400 cells per sample were determined with the NSURFX software (J. L. Vonesch, Institut de Génétique et de Biologie Moléculaire et Cellulaire Illkirch). The distribution of the cell size is shown in the corresponding graphs to the right. Arrows point to small adipocytes and preadipocytes or poorly differentiated adipocytes in e and f, respectively.
Figure 4
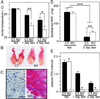
Impaired lipolysis in RXRαadL−/− mice during fasting. (A) Fasted RXRαadL−/− mice suffer from hypothermia. Rectal temperature was determined on 10-week-old CT and RXRαadL−/− (KO) mice at the beginning of the light cycle (fed state) and after a 1- or 2-day fast that was started at the beginning of the light cycle. (Error bars, SEM.) *, P < 0.05; **,P < 0.001. (B) Gross appearance of epididymal fat pad after fasting. Testes and epididymal fat pads from 3-month-old CT and RXRαadL−/− (KO) mice after a 2-day fast are presented. (C) Histological analysis of epididymal fat pad. Sections of the epididymal fat pads shown in_B_ were stained with Oil red O. (Scale bar, 20 μm.) (D) Weight of epididymal fat pad. Epididymal fat pad weight of fed and fasted (2 days) 10-week-old CT and KO mice was measured. Values are expressed as the mean ± SEM (n = 9–10). **,P < 0.001; ***,P < 0.0001. (E) Plasma FFA concentrations. FFA was determined in 10-week-old fed and fasted (1 and 2 days) CT and KO mice. Values are expressed as the mean ± SEM (n = 9–10). # indicates values below background levels (dashed line, 0.1 mmol/liter).
Similar articles
- Functional role of RXRs and PPARgamma in mature adipocytes.
Metzger D, Imai T, Jiang M, Takukawa R, Desvergne B, Wahli W, Chambon P. Metzger D, et al. Prostaglandins Leukot Essent Fatty Acids. 2005 Jul;73(1):51-8. doi: 10.1016/j.plefa.2005.04.007. Prostaglandins Leukot Essent Fatty Acids. 2005. PMID: 15936932 - Tamoxifen-inducible Cre-mediated recombination in adipocytes.
Sassmann A, Offermanns S, Wettschureck N. Sassmann A, et al. Genesis. 2010 Oct 1;48(10):618-25. doi: 10.1002/dvg.20665. Genesis. 2010. PMID: 20715175 - Cre Recombinase Strains Used for the Study of Adipose Tissues and Adipocyte Progenitors.
Liu J, Xu Z, Wu W, Wang Y, Shan T. Liu J, et al. J Cell Physiol. 2017 Oct;232(10):2698-2703. doi: 10.1002/jcp.25675. Epub 2017 Apr 10. J Cell Physiol. 2017. PMID: 27808422 Review.
Cited by
- Slow growth and unstable ribosomal RNA lacking pseudouridine in mouse embryonic fibroblast cells expressing catalytically inactive dyskerin.
Gu BW, Ge J, Fan JM, Bessler M, Mason PJ. Gu BW, et al. FEBS Lett. 2013 Jul 11;587(14):2112-7. doi: 10.1016/j.febslet.2013.05.028. Epub 2013 May 28. FEBS Lett. 2013. PMID: 23726835 Free PMC article. - Regulation of systemic energy homeostasis by serotonin in adipose tissues.
Oh CM, Namkung J, Go Y, Shong KE, Kim K, Kim H, Park BY, Lee HW, Jeon YH, Song J, Shong M, Yadav VK, Karsenty G, Kajimura S, Lee IK, Park S, Kim H. Oh CM, et al. Nat Commun. 2015 Apr 13;6:6794. doi: 10.1038/ncomms7794. Nat Commun. 2015. PMID: 25864946 Free PMC article. - Conditional disruption of the peroxisome proliferator-activated receptor gamma gene in mice results in lowered expression of ABCA1, ABCG1, and apoE in macrophages and reduced cholesterol efflux.
Akiyama TE, Sakai S, Lambert G, Nicol CJ, Matsusue K, Pimprale S, Lee YH, Ricote M, Glass CK, Brewer HB Jr, Gonzalez FJ. Akiyama TE, et al. Mol Cell Biol. 2002 Apr;22(8):2607-19. doi: 10.1128/MCB.22.8.2607-2619.2002. Mol Cell Biol. 2002. PMID: 11909955 Free PMC article. - Inhibition of RXR and PPARgamma ameliorates diet-induced obesity and type 2 diabetes.
Yamauchi T, Waki H, Kamon J, Murakami K, Motojima K, Komeda K, Miki H, Kubota N, Terauchi Y, Tsuchida A, Tsuboyama-Kasaoka N, Yamauchi N, Ide T, Hori W, Kato S, Fukayama M, Akanuma Y, Ezaki O, Itai A, Nagai R, Kimura S, Tobe K, Kagechika H, Shudo K, Kadowaki T. Yamauchi T, et al. J Clin Invest. 2001 Oct;108(7):1001-13. doi: 10.1172/JCI12864. J Clin Invest. 2001. PMID: 11581301 Free PMC article. - Obesity I: Overview and molecular and biochemical mechanisms.
Lustig RH, Collier D, Kassotis C, Roepke TA, Kim MJ, Blanc E, Barouki R, Bansal A, Cave MC, Chatterjee S, Choudhury M, Gilbertson M, Lagadic-Gossmann D, Howard S, Lind L, Tomlinson CR, Vondracek J, Heindel JJ. Lustig RH, et al. Biochem Pharmacol. 2022 May;199:115012. doi: 10.1016/j.bcp.2022.115012. Epub 2022 Apr 5. Biochem Pharmacol. 2022. PMID: 35393120 Free PMC article. Review.
References
- Spiegelman B M, Flier J S. Cell. 1996;87:377–389. - PubMed
- Mandrup S, Lane M D. J Biol Chem. 1997;272:5367–5370. - PubMed
- Fajas L, Fruchart J C, Auwerx J. Curr Opin Cell Biol. 1998;10:165–173. - PubMed
- Loftus T M, Lane M D. Curr Opin Genet Dev. 1997;7:603–608. - PubMed
- Lowell B B. Cell. 1999;99:239–242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases