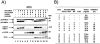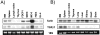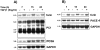Evidence that furin is an authentic transforming growth factor-beta1-converting enzyme - PubMed (original) (raw)
Evidence that furin is an authentic transforming growth factor-beta1-converting enzyme
C M Dubois et al. Am J Pathol. 2001 Jan.
Abstract
Transforming growth factor (TGF)-beta1 plays an essential role in cell growth and differentiation. It is also considered as a gatekeeper of immune homeostasis with gene disruption leading to autoimmune and inflammatory diseases. TGF-beta1 is produced as an inactive precursor polypeptide that can be efficiently secreted but correct proteolytic cleavage is an essential step for its activation. Assessment of the cleavage site has revealed a unique R-H-R-R sequence reminiscent of proprotein convertase (PC) recognition motifs and has previously demonstrated that this PC-like cleavage site is correctly cleaved by furin, a member of the PC family. Here we report that among PC members, furin more closely satisfies the requirements needed to fulfill the role of a genuine TGF-beta1 convertase. Even though six members of the PC family have the ability to cleave TGF-beta1, ectopic expression of alpha(1)-antitrypsin Portland (alpha(1)-AT-PDX), a potent furin inhibitor, blocked 80% of TGF-beta1 processing mediated by endogenous enzymes as demonstrated in an in vitro digestion assay. Genetic complementation of a furin-deficient LoVo cell line with the wild-type gene restores the production of mature and bioactivable TGF-beta1. Moreover, both furin and TGF-beta are coordinately expressed and regulated in vitro and in vivo in the hematopoietic and immune system, an important tissue target. These results demonstrate for the first time that furin is an authentic and adaptive TGF-beta1-converting enzyme whereas other members of the PC family might substitute or supplement furin activity. Our study advances our comprehension of the complexity of the TGF-beta system and should facilitate the development of therapeutically useful TGF-beta inhibitors.
Figures
Figure 1.
A: Processing of TGF-β1 precursor by PCs in LoVo cells. LoVo cells were infected with vaccinia recombinants for both the TGF-β1 precursor (VV: TGF-β) or an unrelated control vaccinia recombinant (VV: POMC) and one of the PCs (PC1/PC3, VV: PC1; PC2, VV: PC2; PC5A, VV: PC5A; furin, VV: FUR, PACE-4, VV: PACE-4; PC5B, VV: PC5B; PC7, VV: PC7) at a multiplicity of infection of 5. Eighteen hours after infection, supernates or cell lysates were electrophoresed on 12% SDS-PAGE gels under reducing conditions. Immunoblots were performed with an anti-human LAP IgG (revealing an ∼55-kd proTGF-β1 and a 40-kd proregion forms) or PAN-specific anti-TGF-β antibodies (revealing an ∼12-kd mature TGF-β1 form). A representative experiment out of four performed is shown. B: Measure of TGF-β1 in cell supernatants or cell lysates. Eighteen hours after cell infection, LoVo cell supernates were collected, heat-activated (80°C, 5 minutes), and used to measure bioactive TGF-β1. A representative experiment out of four performed is shown.
Figure 2.
A: Processing of TGF-β1 precursor by PCs in BSC-40 cells. Cells were infected with vaccinia recombinants for both the TGF-β1 precursor (VV: TGF-β) or an unrelated control vaccinia recombinant (VV: POMC) and one of the PCs (PC1/PC3, VV: PC1; PC2, VV: PC2; PC5A, VV: PC5A; furin, VV: FUR, PACE-4, VV: PACE-4; PC5B, VV: PC5B) at a multiplicity of infection of 5. Eighteen hours after infection, supernates were collected, concentrated, and electrophoresed on 12% SDS-PAGE gels under reducing conditions. Immunoblots were performed with an anti-human LAP IgG (revealing an ∼55-kd proTGF-β1 and an ∼40-kd proregion forms) or PAN-specific anti-TGF-β antibodies (revealing an ∼12-kd mature TGF-β1 form). A representative experiment out of three performed is shown. B: Measure of TGF-β1 in cell supernates. Eighteen hours after cell infection, cell supernates were collected, heat-activated (80°C, 5 minutes), and used to measure bioactive TGF-β1. A representative experiment out of three performed is shown.
Figure 3.
A: Inhibition of TGF-β1 processing by α1-PDX. BSC-40 cells were infected with vaccinia recombinants for TGF-β1 precursor (VV: TGF-β), control vaccinia virus (VV: WT), furin-encoding vaccinia virus (VV: FUR) and α1-PDX-encoding vaccinia virus (VV: α1-PDX) at the indicated multiplicity of infection. Eighteen hours after cell infection, cell supernates were collected and electrophoresed on 12% SDS-PAGE gels under reducing conditions. Immunoblots were performed with an anti-human LAP, PAN-specific anti-TGF-β antibodies, furin-specific antisera (revealing an ∼95-kd form) and α1-antitrypsin-specific antisera (revealing an ∼55-kd α1-PDX form). B: Measure of TGF-β1 in cell supernates. Eighteen hours after cell infection, cell supernates were collected, heat-activated (80°C, 5 minutes), and used to quantitate bioactive TGF-β1 as described in Material and Methods. A representative experiment out of two performed is shown.
Figure 4.
A: Furin and TGF-β1 mRNA expression in hematopoietic/immune cells and tissues. B: Furin and TGF-β1 mRNA expression in mice tissues. Northern blot analysis used total mRNA (5 μg/lane) and a rat TGF-β1 or a rat furin riboprobe. Ethidium bromide staining of 18S is shown as a control for mRNA integrity.
Figure 5.
PC mRNA regulation by TG F-β1. The rat insulinoma cell line (Rin m5F) (A) or rat fibroblastic kidney cell line (NRK-49F) (B) were incubated for 7, 15, and 24 hours in the presence or absence of 5 ng of recombinant human TGF-β1. Total mRNAs (10 μg/lane) were probed with rat riboprobes specific for furin, PC1, PC5A/PC5B, PACE4, or GAPDH cDNA.
Figure 6.
Co-regulation of furin and TGF-β1 mRNAs. NRK-49F cells were incubated for 4, 6, or 8 hours in the presence or absence of 10 μmol/L PMA or 5 ng/ml human recombinant TGF-β1. Total mRNAs (10 μg/lane) were probed with rat riboprobe specific for furin, TGF-β1, PC7, and a GAPDH cDNA.
Similar articles
- Processing of immunosuppressive pro-TGF-beta 1,2 by human glioblastoma cells involves cytoplasmic and secreted furin-like proteases.
Leitlein J, Aulwurm S, Waltereit R, Naumann U, Wagenknecht B, Garten W, Weller M, Platten M. Leitlein J, et al. J Immunol. 2001 Jun 15;166(12):7238-43. doi: 10.4049/jimmunol.166.12.7238. J Immunol. 2001. PMID: 11390472 - Processing of transforming growth factor beta 1 precursor by human furin convertase.
Dubois CM, Laprise MH, Blanchette F, Gentry LE, Leduc R. Dubois CM, et al. J Biol Chem. 1995 May 5;270(18):10618-24. doi: 10.1074/jbc.270.18.10618. J Biol Chem. 1995. PMID: 7737999 - Endoproteolytic processing of integrin pro-alpha subunits involves the redundant function of furin and proprotein convertase (PC) 5A, but not paired basic amino acid converting enzyme (PACE) 4, PC5B or PC7.
Lissitzky JC, Luis J, Munzer JS, Benjannet S, Parat F, Chrétien M, Marvaldi J, Seidah NG. Lissitzky JC, et al. Biochem J. 2000 Feb 15;346 Pt 1(Pt 1):133-8. Biochem J. 2000. PMID: 10657249 Free PMC article. - Structure and function of eukaryotic proprotein processing enzymes of the subtilisin family of serine proteases.
Van de Ven WJ, Roebroek AJ, Van Duijnhoven HL. Van de Ven WJ, et al. Crit Rev Oncog. 1993;4(2):115-36. Crit Rev Oncog. 1993. PMID: 8420571 Review. - Furin: a mammalian subtilisin/Kex2p-like endoprotease involved in processing of a wide variety of precursor proteins.
Nakayama K. Nakayama K. Biochem J. 1997 Nov 1;327 ( Pt 3)(Pt 3):625-35. doi: 10.1042/bj3270625. Biochem J. 1997. PMID: 9599222 Free PMC article. Review.
Cited by
- Cyclical strain modulates metalloprotease and matrix gene expression in human tenocytes via activation of TGFβ.
Jones ER, Jones GC, Legerlotz K, Riley GP. Jones ER, et al. Biochim Biophys Acta. 2013 Dec;1833(12):2596-2607. doi: 10.1016/j.bbamcr.2013.06.019. Epub 2013 Jul 2. Biochim Biophys Acta. 2013. PMID: 23830915 Free PMC article. - Rab9-dependent retrograde transport and endosomal sorting of the endopeptidase furin.
Chia PZ, Gasnereau I, Lieu ZZ, Gleeson PA. Chia PZ, et al. J Cell Sci. 2011 Jul 15;124(Pt 14):2401-13. doi: 10.1242/jcs.083782. Epub 2011 Jun 21. J Cell Sci. 2011. PMID: 21693586 Free PMC article. - Bone Morphogenetic Protein 2/4 in Mollusk, Haliotis diversicolor: Its Expression and Osteoinductive Function In Vitro.
Suwannasing C, Buddawong A, Khumpune S, Habuddha V, Weerachatyanukul W, Asuvapongpatana S. Suwannasing C, et al. Mar Biotechnol (NY). 2021 Oct;23(5):836-846. doi: 10.1007/s10126-021-10071-2. Epub 2021 Oct 5. Mar Biotechnol (NY). 2021. PMID: 34609689 - Proprotein convertase inhibition: Paralyzing the cell's master switches.
Klein-Szanto AJ, Bassi DE. Klein-Szanto AJ, et al. Biochem Pharmacol. 2017 Sep 15;140:8-15. doi: 10.1016/j.bcp.2017.04.027. Epub 2017 Apr 27. Biochem Pharmacol. 2017. PMID: 28456517 Free PMC article. Review. - microRNA-221 Inhibits Latent TGF-β1 Activation through Targeting Thrombospondin-1 to Attenuate Kidney Failure-Induced Cardiac Fibrosis.
Zhou Y, Ng DYE, Richards AM, Wang P. Zhou Y, et al. Mol Ther Nucleic Acids. 2020 Oct 4;22:803-814. doi: 10.1016/j.omtn.2020.09.041. eCollection 2020 Dec 4. Mol Ther Nucleic Acids. 2020. PMID: 33230477 Free PMC article.
References
- Assoian RK, Komoriya A, Meyers CA, Miller DM, Sporn MB: Transforming growth factor-beta in human platelets. Identification of a major storage site, purification, and characterization. J Biol Chem 1983, 258:7155-7160 - PubMed
- Roberts AB, Flanders KC, Kondaiah P, Thompson NL, Van Obberghen-Schilling E, Wakefield L, Rossi P, de Crombrugghe B, Heine U, Sporn MB: Transforming growth factor β: biochemistry and roles in embryogenesis, tissue repair and remodeling, and carcinogenesis. (Review). Recent Prog Horm Res 1988, 44:157-197 - PubMed
- Kingsley DM: The TGF-β superfamily: new members, new receptors, and new genetic tests of function in different organisms. Genes Dev 1994, 8:133-146 - PubMed
- Derynck R, Jarrett JA, Chen EY, Eaton DH, Bell JR, Assoian RK, Roberts AB, Sporn MB, Goeddel DV: Human transforming growth factor-β complementary DNA sequence and expression in normal and transformed cells. Nature 1985, 316:701-705 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases