Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection - PubMed (original) (raw)
Comparative Study
. 2001 Jan 15;193(2):181-94.
doi: 10.1084/jem.193.2.181.
M A Altfeld, E S Rosenberg, T Nguyen, Y Tang, R L Eldridge, M M Addo, S He, J S Mukherjee, M N Phillips, M Bunce, S A Kalams, R P Sekaly, B D Walker, C Brander
Affiliations
- PMID: 11148222
- PMCID: PMC2193346
- DOI: 10.1084/jem.193.2.181
Comparative Study
Substantial differences in specificity of HIV-specific cytotoxic T cells in acute and chronic HIV infection
P J Goulder et al. J Exp Med. 2001.
Abstract
Cytotoxic T lymphocytes (CTLs) play a vital part in controlling viral replication during human viral infections. Most studies in human infections have focused on CTL specificities in chronic infection and few data exist regarding the specificity of the initial CTL response induced in acute infection. In this study, HIV-1 infection in persons expressing human histocompatibility leukocyte antigen (HLA)-A*0201 was used as a means of addressing this issue. In chronic infection, the dominant HLA-A*0201-restricted CTL response is directed towards the epitope SLYNTVATL ("SL9") in p17 Gag (residues 77-85). This epitope is targeted by 75% of HLA-A*0201-positive adults, and the magnitude of this A*0201-SL9 response shows a strong negative association with viral load in progressive infection. Despite using the highly sensitive peptide-major histocompatibility complex tetramer and intracellular cytokine assays, responses to the SL9 epitope were not detectable in any of 11 HLA-A*0201-positive subjects with acute HIV-1 infection (P = 2 x 10(-6)), even when assays were repeated using the SL9 peptide variant that was encoded by their autologous virus. In contrast, multiple responses (median 3) to other epitopes were evident in 7 of the 11 A*0201-positive subjects. Longitudinal study of two subjects confirmed that the A*0201-SL9 response emerged later than other CTL responses, and after viral set point had been reached. Together, these data show that the CTL responses that are present and that even may dominate in chronic infection may differ substantially from those that constitute the initial antiviral CTL response. This finding is an important consideration in vaccine design and in the evaluation of vaccine candidates.
Figures
Figure 1
(A) Characterization of the HIV-specific CTL responses made in acute HIV infection. The method used is illustrated for subject AC01. Overlapping peptides spanning p17 Gag, p24 Gag, Nef, RT, gp120, gp41, Rev, and Tat were used in Elispot assays, as well as published optimal peptides presented by HLA-A*0201, A3, B35, or Cw4. Examples of positive and negative responses are shown. No responses to Tat or Rev overlapping peptides were observed in this subject (not shown). SFC, spot-forming cell. (B) Proportion of A*0201-positive subjects in acute infection showing a detectable response to the A*0201-SLYNTVATL epitope. (C) Proportion of A*0201-positive subjects in chronic infection showing a detectable response to the A*0201-SLYNTVATL epitope, using data from four published studies. Criteria for inclusion of a published study were: demonstration of specificity of A*0201-SL9 response by cytotoxicity assays or peptide–MHC tetramers, and more than 1 subject studied.
Figure 1
(A) Characterization of the HIV-specific CTL responses made in acute HIV infection. The method used is illustrated for subject AC01. Overlapping peptides spanning p17 Gag, p24 Gag, Nef, RT, gp120, gp41, Rev, and Tat were used in Elispot assays, as well as published optimal peptides presented by HLA-A*0201, A3, B35, or Cw4. Examples of positive and negative responses are shown. No responses to Tat or Rev overlapping peptides were observed in this subject (not shown). SFC, spot-forming cell. (B) Proportion of A*0201-positive subjects in acute infection showing a detectable response to the A*0201-SLYNTVATL epitope. (C) Proportion of A*0201-positive subjects in chronic infection showing a detectable response to the A*0201-SLYNTVATL epitope, using data from four published studies. Criteria for inclusion of a published study were: demonstration of specificity of A*0201-SL9 response by cytotoxicity assays or peptide–MHC tetramers, and more than 1 subject studied.
Figure 1
(A) Characterization of the HIV-specific CTL responses made in acute HIV infection. The method used is illustrated for subject AC01. Overlapping peptides spanning p17 Gag, p24 Gag, Nef, RT, gp120, gp41, Rev, and Tat were used in Elispot assays, as well as published optimal peptides presented by HLA-A*0201, A3, B35, or Cw4. Examples of positive and negative responses are shown. No responses to Tat or Rev overlapping peptides were observed in this subject (not shown). SFC, spot-forming cell. (B) Proportion of A*0201-positive subjects in acute infection showing a detectable response to the A*0201-SLYNTVATL epitope. (C) Proportion of A*0201-positive subjects in chronic infection showing a detectable response to the A*0201-SLYNTVATL epitope, using data from four published studies. Criteria for inclusion of a published study were: demonstration of specificity of A*0201-SL9 response by cytotoxicity assays or peptide–MHC tetramers, and more than 1 subject studied.
Figure 2
Recognition of SLYNTVATL only in A*0201-positive subjects in chronic infection. Intracellular staining using SL9 and a positive control HIV peptide epitope and A*0201-SL9 tetramer staining of PBMCs from chronically infected subject 9354 (HLA-A*0201/3 B7/35 Cw4/7) and 2 of the 11 A*0201-positive subjects studied in acute infection (expressed as percentage of CD8+ T cells). Controls: percentage of CD8+ T cells showing intracellular IFN-γ staining after incubation with no peptide was, respectively, 0.00% (subject 9354), 0.03% (AC29), and 0.02% (AC13; data not shown). The HIV peptides used as positive controls had previously been established as recognized by these subjects: B7 Gag TL9, TPQDLNTML (p24 Gag); B8 Nef FL8, FLKEKGGL; and B14-gp41-EL9, ERYLKDQQL (reference 50).
Figure 3
(A and B) Recognition of SLYNTVATL and autologous SL9 variant in Elispot assays. (A) Positive control chronic subject 161j, recognition of SL9, and autologous variant SL
F
NTVATL (reference 45). (B) Acute subject AC04: no recognition either of SL9 or autologous SL9 variant. Data from the other 10 A*0201 subjects in acute infection are not shown. SFC, spot-forming cell. (C–H) Recognition of SL9 and SL9 variants that arise most often in HIV infection (>90% of published B clade United States originating sequences; reference 50) using CTL clones derived from PBMCs from chronically infected A*0201-positive subjects.
Figure 3
(A and B) Recognition of SLYNTVATL and autologous SL9 variant in Elispot assays. (A) Positive control chronic subject 161j, recognition of SL9, and autologous variant SL
F
NTVATL (reference 45). (B) Acute subject AC04: no recognition either of SL9 or autologous SL9 variant. Data from the other 10 A*0201 subjects in acute infection are not shown. SFC, spot-forming cell. (C–H) Recognition of SL9 and SL9 variants that arise most often in HIV infection (>90% of published B clade United States originating sequences; reference 50) using CTL clones derived from PBMCs from chronically infected A*0201-positive subjects.
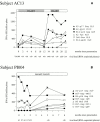
Figure 4
(A and B) Longitudinal study of HIV-specific epitope recognition in subject AC13 (presenting preseroconversion) and subject PI004 (presenting 7–28 wk after seroconversion). Before the onset of HAART in subject 5192d, the viral load had declined from 730,000 HIV-1 RNA copies/ml plasma to 5,000 copies/ml plasma. Subject PI004 did not receive antiretroviral therapy at any time. Peptides that were recognized (references 50 and 76): A2-p17 Gag SL9, SLYNTVATL; A2-gp41 SV10, SLLNATAIAV; B14-p24 Gag DA9, DRFYKTLRA; A2 Vpr, AIIRILQQL; Cw5-Rev SL9 SAEPVPLQL, B14-gp41 ERYLKDQQL; B57-Gag TW10, TSTLQEQIGW; B57-Gag IW9, ISPRTLNAW; B57-Gag KF11, KAFSPEVIPMF; B57-Gag QW9, QASQEVKNW; B8-p24 Gag EI8, EIYKRWII; and B8 Nef FL8, FLKEKGGL. SFC, spot-forming cell.
Similar articles
- The HIV-1 HLA-A2-SLYNTVATL is a help-independent CTL epitope.
Kan-Mitchell J, Bisikirska B, Wong-Staal F, Schaubert KL, Bajcz M, Bereta M. Kan-Mitchell J, et al. J Immunol. 2004 May 1;172(9):5249-61. doi: 10.4049/jimmunol.172.9.5249. J Immunol. 2004. PMID: 15100263 - Absence of immunodominant anti-Gag p17 (SL9) responses among Gag CTL-positive, HIV-uninfected vaccine recipients expressing the HLA-A*0201 allele.
Ferrari G, Neal W, Ottinger J, Jones AM, Edwards BH, Goepfert P, Betts MR, Koup RA, Buchbinder S, McElrath MJ, Tartaglia J, Weinhold KJ. Ferrari G, et al. J Immunol. 2004 Aug 1;173(3):2126-33. doi: 10.4049/jimmunol.173.3.2126. J Immunol. 2004. PMID: 15265949 - Efficient processing of the immunodominant, HLA-A*0201-restricted human immunodeficiency virus type 1 cytotoxic T-lymphocyte epitope despite multiple variations in the epitope flanking sequences.
Brander C, Yang OO, Jones NG, Lee Y, Goulder P, Johnson RP, Trocha A, Colbert D, Hay C, Buchbinder S, Bergmann CC, Zweerink HJ, Wolinsky S, Blattner WA, Kalams SA, Walker BD. Brander C, et al. J Virol. 1999 Dec;73(12):10191-8. doi: 10.1128/JVI.73.12.10191-10198.1999. J Virol. 1999. PMID: 10559335 Free PMC article. - Mother-to-child transmission of HIV infection and CTL escape through HLA-A2-SLYNTVATL epitope sequence variation.
Goulder PJ, Pasquier C, Holmes EC, Liang B, Tang Y, Izopet J, Saune K, Rosenberg ES, Burchett SK, McIntosh K, Barnardo M, Bunce M, Walker BD, Brander C, Phillips RE. Goulder PJ, et al. Immunol Lett. 2001 Nov 1;79(1-2):109-16. doi: 10.1016/s0165-2478(01)00272-3. Immunol Lett. 2001. PMID: 11595297 - Recognition patterns of HLA-A2-restricted human immunodeficiency virus-1-specific cytotoxic T-lymphocytes in a cohort of HIV-1-infected individuals.
Schmitt-Haendle M, Bachmann O, Harrer E, Schmidt B, Bäuerle M, Harrer T. Schmitt-Haendle M, et al. Viral Immunol. 2005;18(4):627-36. doi: 10.1089/vim.2005.18.627. Viral Immunol. 2005. PMID: 16359229
Cited by
- Vaccine design for CD8 T lymphocyte responses.
Koup RA, Douek DC. Koup RA, et al. Cold Spring Harb Perspect Med. 2011 Sep;1(1):a007252. doi: 10.1101/cshperspect.a007252. Cold Spring Harb Perspect Med. 2011. PMID: 22229122 Free PMC article. Review. - Conserved HIV-1 epitopes continuously elicit subdominant cytotoxic T-lymphocyte responses.
Liu Y, McNevin J, Rolland M, Zhao H, Deng W, Maenza J, Stevens CE, Collier AC, McElrath MJ, Mullins JI. Liu Y, et al. J Infect Dis. 2009 Dec 15;200(12):1825-33. doi: 10.1086/648401. J Infect Dis. 2009. PMID: 19909083 Free PMC article. - Cross-reactivity between HLA-A2-restricted FLU-M1:58-66 and HIV p17 GAG:77-85 epitopes in HIV-infected and uninfected individuals.
Acierno PM, Newton DA, Brown EA, Maes LA, Baatz JE, Gattoni-Celli S. Acierno PM, et al. J Transl Med. 2003 Aug 14;1(1):3. doi: 10.1186/1479-5876-1-3. J Transl Med. 2003. PMID: 14527342 Free PMC article. - CD4 responses to conserved HIV-1 T helper epitopes show both negative and positive associations with virus load in chronically infected subjects.
Boaz MJ, Waters A, Murad S, Easterbrook PJ, D'Sousa E, van Wheeley C, Vyakarnam A. Boaz MJ, et al. Clin Exp Immunol. 2003 Dec;134(3):454-63. doi: 10.1111/j.1365-2249.2003.02307.x. Clin Exp Immunol. 2003. PMID: 14632751 Free PMC article. - Fusion of ubiquitin to HIV gag impairs human monocyte-derived dendritic cell maturation and reduces ability to induce gag T cell responses.
Herath S, Benlahrech A, Papagatsias T, Athanasopoulos T, Bouzeboudjen Z, Hervouet C, Klavinskis L, Meiser A, Kelleher P, Dickson G, Patterson S. Herath S, et al. PLoS One. 2014 Feb 5;9(2):e88327. doi: 10.1371/journal.pone.0088327. eCollection 2014. PLoS One. 2014. PMID: 24505475 Free PMC article.
References
- Riddell S.R., Watanabe K.S., Goodrich J.M., Agha M.E., Greenberg P.D. Restoration of viral immunity in immunodeficient humans by the adoptive transfer of CTL clones. Science. 1992;257:238–241. - PubMed
- Rickinson A.B., Moss D. Human cytotoxic T lymphocyte responses to Epstein-Barr virus infection. Annu. Rev. Immunol. 1997;15:405–431. - PubMed
- Maini M.K., Boni C., Ogg G., King A.S., Reignat S., Lee C.K., Larrubia J.R., Webster G.J., McMichael A.J., Ferrari C. Direct ex vivo analysis of hepatitis B virus-specific CD8+ T cells associated with the control of infection. Gastroenterology. 1999;117:1386–1396. - PubMed
- Goulder P.J.R, Rowland-Jones S., McMichael A.J., Walker B.D. Anti-human immunodeficiency virus cellular immunityprogress towards vaccine design AIDS. 13Suppl. A1999. S121 S136 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI01541/AI/NIAID NIH HHS/United States
- AI46995/AI/NIAID NIH HHS/United States
- R37 AI028568/AI/NIAID NIH HHS/United States
- R01 AI046995/AI/NIAID NIH HHS/United States
- R01 AI028568/AI/NIAID NIH HHS/United States
- R01 AI030914/AI/NIAID NIH HHS/United States
- R01 AI039966/AI/NIAID NIH HHS/United States
- AI28568/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials