Nuclear localization of NPR1 is required for activation of PR gene expression - PubMed (original) (raw)
Nuclear localization of NPR1 is required for activation of PR gene expression
M Kinkema et al. Plant Cell. 2000 Dec.
Abstract
Systemic acquired resistance (SAR) is a broad-spectrum resistance in plants that involves the upregulation of a battery of pathogenesis-related (PR) genes. NPR1 is a key regulator in the signal transduction pathway that leads to SAR. Mutations in NPR1 result in a failure to induce PR genes in systemic tissues and a heightened susceptibility to pathogen infection, whereas overexpression of the NPR1 protein leads to increased induction of the PR genes and enhanced disease resistance. We analyzed the subcellular localization of NPR1 to gain insight into the mechanism by which this protein regulates SAR. An NPR1-green fluorescent protein fusion protein, which functions the same as the endogenous NPR1 protein, was shown to accumulate in the nucleus in response to activators of SAR. To control the nuclear transport of NPR1, we made a fusion of NPR1 with the glucocorticoid receptor hormone binding domain. Using this steroid-inducible system, we clearly demonstrate that nuclear localization of NPR1 is essential for its activity in inducing PR genes.
Figures
Figure 1.
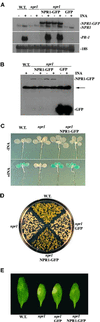
Complementation of the npr1 Mutant Phenotypes by the NPR1-GFP Fusion Protein. (A) Gel blot of RNA (20 μg) from wild type (W.T.), npr1, two independent 35S::NPR1-GFP transformants (NPR1-GFP; in npr1), and a 35S::GFP transformant (GFP; in npr1). Seedlings were grown for 8 days on MS medium with (+) or without (−) 0.1 mM INA. The blot was probed for NPR1, PR-1, and the 18S rRNA. (B) Gel blot of protein (100 μg) from wild type, two independent 35S::NPR1-GFP transformants (in npr1), and a 35S::GFP transformant (in npr1). Seedlings were grown for 8 days on MS medium with or without 0.1 mM INA. The blot was probed with antibodies against GFP. The arrow indicates a cross-reacting nonspecific protein. (C) BGL2::GUS expression in wild type, npr1, and two independent 35S::NPR1-GFP transformants (in npr1) grown for 13 days on MS medium with or without 0.1 mM INA. (D) Growth of wild type, npr1, a 35S::NPR1-GFP transformant (in npr1), and a 35S::GFP transformant (in npr1) on MS medium containing 0.5 mM SA. The image was made after 11 days. (E) Symptoms on leaves infected with Psm ES4326. The left halves of leaves from 4-week-old plants were infected with Psm ES4326 (). Representative images from wild type, npr1, a 35S::GFP transformant (in npr1), and a 35S::NPR1-GFP transformant (in npr1) were made 4 days after infection.
Figure 2.
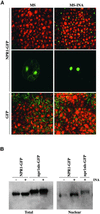
Nuclear Localization of NPR1-GFP in Response to SAR Induction. (A) Confocal images of GFP fluorescence in mesophyll cells (top and bottom pairs of images) and in guard cells (middle pair of images) of cotyledons from 7-day-old seedlings grown on MS or MS-INA. For mesophyll cells, GFP fluorescence is shown in the green channel and differential interference contrast images are shown in the red channel. (B) Gel blot of total protein (100 μg) and the nuclear-fractionated protein (12 μg). Protein was isolated from transgenic seedlings grown for 10 days on MS medium with (+) or without (−) 0.1 mM INA. The blot was probed with antibodies against GFP. The cytoplasmic npr1nls-GFP protein serving as a control indicates that the nuclear fraction contains relatively little cytoplasmic contamination.
Figure 3.
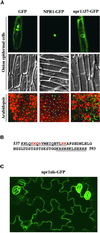
Identification of the NLS in NPR1 by Mutagenesis. (A) Subcellular localization of GFP, NPR1-GFP, and npr1Δ57-GFP (a mutant lacking the C-terminal 57 amino acids of NPR1). GFP fluorescence (top images) and differential interference contrast images (middle images) of onion epidermal cells were compared to show the subcellular localization of GFP (cytoplasmic and nuclear), NPR1-GFP (nuclear), and npr1Δ57-GFP (cytoplasmic). Confocal GFP images (bottom images) were captured from 7-day-old transgenic Arabidopsis seedlings expressing GFP, NPR1-GFP, or npr1Δ57-GFP grown on MS-INA. GFP fluorescence is shown in the green channel; differential interference contrast images are shown in the red channel. (B) Sequence of the C-terminal 57 amino acids of NPR1. The two potential NLSs are underlined. Point mutations in the amino acids shown in red had a marked effect on NPR1-GFP nuclear localization, whereas mutations in the amino acids shown in italics had no detectable effect on nuclear localization. Mutations in all five amino acids shown in red resulted in the exclusive cytoplasmic localization of the fusion protein npr1nls-GFP. (C) Cytoplasmic localization of npr1nls-GFP in leaf epidermal cells of transgenic seedlings.
Figure 4.
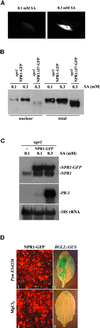
Increased Nuclear Accumulation of NPR1-GFP Correlates with Increased Expression of PR Genes. (A) Representative images of nuclear NPR1-GFP fluorescence in leaf mesophyll cells of seedlings grown for 12 days on MS medium containing either 0.1 or 0.3 mM SA. (B) Gel blot of total protein (40 μg) and the nuclear-fractionated protein (20 μg). Protein was isolated from transgenic seedlings 35S::NPR1-GFP or 35S::_npr1_Δ57-GFP (NPR1-GFP or npr1Δ57-GFP) grown for 12 days on MS medium containing either 0.1 or 0.3 mM SA. The blot was probed with antibodies against GFP. The cytoplasmic npr1Δ57-GFP protein served as a control, indicating that the nuclear fraction contains relatively little cytoplasmic contamination. (C) Gel blot of RNA from npr1 and an npr1 line expressing NPR1-GFP. Seedlings were grown for 12 days on MS medium containing either 0.1 or 0.3 mM SA. The blot was probed for NPR1, PR-1, and 18S rRNA. (D) GFP fluorescence and BGL2::GUS expression in a 35S::NPR1-GFP transformant (in npr1) after infiltration with either Psm ES4326 ( in 10 mM MgCl2) or 10 mM MgCl2. Leaves were infected on their left halves, and representative GFP fluorescence images from the infected halves of the leaves are shown. The expression of BGL2::GUS then was examined as described previously (Cao et al., 1994). All images were made 3 days after treatment.
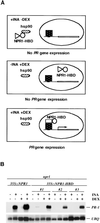
Figure 5.
Nuclear Localization of NPR1 Is Essential for Its Function in Activating PR-1 Gene Expression. (A) Strategy used to control the nuclear localization of NPR1. (B) Gel blot of RNA (10 μg) from 35S::NPR1 (in npr1) and three independent 35S::NPR1-HBD transformants (in npr1). Seedlings were grown for 14 days on MS medium with (+) or without (−) DEX (5 μM) and with or without INA (20 μM). The blot was probed for PR-1 and ubiquitin (UBQ) mRNA.
Similar articles
- Identification and cloning of a negative regulator of systemic acquired resistance, SNI1, through a screen for suppressors of npr1-1.
Li X, Zhang Y, Clarke JD, Li Y, Dong X. Li X, et al. Cell. 1999 Aug 6;98(3):329-39. doi: 10.1016/s0092-8674(00)81962-5. Cell. 1999. PMID: 10458608 - Evidence for a disease-resistance pathway in rice similar to the NPR1-mediated signaling pathway in Arabidopsis.
Chern MS, Fitzgerald HA, Yadav RC, Canlas PE, Dong X, Ronald PC. Chern MS, et al. Plant J. 2001 Jul;27(2):101-13. doi: 10.1046/j.1365-313x.2001.01070.x. Plant J. 2001. PMID: 11489188 - The Arabidopsis NPR1/NIM1 protein enhances the DNA binding activity of a subgroup of the TGA family of bZIP transcription factors.
Després C, DeLong C, Glaze S, Liu E, Fobert PR. Després C, et al. Plant Cell. 2000 Feb;12(2):279-90. Plant Cell. 2000. PMID: 10662863 Free PMC article. - [Nonexpressor of pathogenesis-related genes 1 (NPR1): a key node of plant disease resistance signalling network].
Zhang HZ, Cai XZ. Zhang HZ, et al. Sheng Wu Gong Cheng Xue Bao. 2005 Jul;21(4):511-5. Sheng Wu Gong Cheng Xue Bao. 2005. PMID: 16176083 Review. Chinese. - Systemic acquired resistance: turning local infection into global defense.
Fu ZQ, Dong X. Fu ZQ, et al. Annu Rev Plant Biol. 2013;64:839-63. doi: 10.1146/annurev-arplant-042811-105606. Epub 2013 Jan 25. Annu Rev Plant Biol. 2013. PMID: 23373699 Review.
Cited by
- Isolation of Catharanthus roseus (L.) G. Don Nuclei and Measurement of Rate of Tryptophan decarboxylase Gene Transcription Using Nuclear Run-On Transcription Assay.
Kumar S, Bhatia S. Kumar S, et al. PLoS One. 2015 May 29;10(5):e0127892. doi: 10.1371/journal.pone.0127892. eCollection 2015. PLoS One. 2015. PMID: 26024519 Free PMC article. - Tobacco TTG2 regulates vegetative growth and seed production via the predominant role of ARF8 in cooperation with ARF17 and ARF19.
Ge J, Li B, Shen D, Xie J, Long J, Dong H. Ge J, et al. BMC Plant Biol. 2016 Jun 2;16(1):126. doi: 10.1186/s12870-016-0815-3. BMC Plant Biol. 2016. PMID: 27255279 Free PMC article. - Protein poly(ADP-ribosyl)ation regulates arabidopsis immune gene expression and defense responses.
Feng B, Liu C, de Oliveira MV, Intorne AC, Li B, Babilonia K, de Souza Filho GA, Shan L, He P. Feng B, et al. PLoS Genet. 2015 Jan 8;11(1):e1004936. doi: 10.1371/journal.pgen.1004936. eCollection 2015 Jan. PLoS Genet. 2015. PMID: 25569773 Free PMC article. - AtWAKL10, a Cell Wall Associated Receptor-Like Kinase, Negatively Regulates Leaf Senescence in Arabidopsis thaliana.
Li L, Li K, Ali A, Guo Y. Li L, et al. Int J Mol Sci. 2021 May 5;22(9):4885. doi: 10.3390/ijms22094885. Int J Mol Sci. 2021. PMID: 34063046 Free PMC article. - EDS1-interacting J protein 1 is an essential negative regulator of plant innate immunity in Arabidopsis.
Liu H, Li Y, Hu Y, Yang Y, Zhang W, He M, Li X, Zhang C, Kong F, Liu X, Hou X. Liu H, et al. Plant Cell. 2021 Mar 22;33(1):153-171. doi: 10.1093/plcell/koaa007. Plant Cell. 2021. PMID: 33751092 Free PMC article.
References
- Alexander, D., Goodman, R.M., Gut-Rella, M., Glascock, C., Weymann, K., Friedrich, L., Maddox, D., Ahl-Goy, P., Luntz, T., Ward, E., and Ryals, J. (1993). Increased tolerance to two oomycete pathogens in transgenic tobacco expressing pathogenesis-related protein 1a. Proc. Natl. Acad. Sci. USA 90 7327–7331. - PMC - PubMed
- Aoyama, T., and Chua, N.-H. (1997). A glucocorticoid-mediated transcriptional induction system in transgenic plants. Plant J. 11 605–612. - PubMed
- Aravind, L., and Koonin, E.V. (1999). Fold prediction and evolutionary analysis of the POZ domain: Structural and evolutionary relationship with the potassium channel tetramerization domain. J. Mol. Biol. 285 1353–1361. - PubMed
- Beato, M. (1989). Gene regulation by steroid hormones. Cell 56 335–344. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous