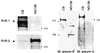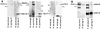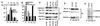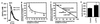Regulating ankyrin dynamics: Roles of sigma-1 receptors - PubMed (original) (raw)
Regulating ankyrin dynamics: Roles of sigma-1 receptors
T Hayashi et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Ankyrin is a cytoskeletal adaptor protein that controls important cellular functions, including Ca(2+) efflux at inositol 1,4,5-trisphosphate receptors (IP(3)R) on the endoplasmic reticulum. The present study found that sigma-1 receptors (Sig-1R), unique endoplasmic reticulum proteins that bind certain steroids, neuroleptics, and psychotropic drugs, form a trimeric complex with ankyrin B and IP(3)R type 3 (IP(3)R-3) in NG-108 cells. The trimeric complex could be coimmunoprecipitated by antibodies against any of the three proteins. Sig-1R agonists such as pregnenolone sulfate and cocaine caused the dissociation of an ankyrin B isoform (ANK 220) from IP(3)R-3. This effect caused by Sig-1R agonists was blocked by a Sig-1R antagonist. The degree of dissociation of ANK 220 from IP(3)R-3 caused by Sig-1R ligands correlates excellently with the ligands' efficacies in potentiating the bradykinin-induced increase in cytosolic free Ca(2+) concentration. Immunocytohistochemistry showed that Sig-1R, ankyrin B, and IP(3)R-3 are colocalized in NG-108 cells in perinuclear areas and in regions of cell-to-cell communication. These results suggest that Sig-1R and associated ligands may play important roles in cells by controlling the function of cytoskeletal proteins and that the Sig-1R/ANK220/IP(3)R-3 complex regulating Ca(2+) signaling may represent a site of action for neurosteroids and cocaine.
Figures
Figure 1
Identification of IP3R-3, ankyrin B in NG-108 cells. IP3R-3 and ankyrin B exist in NG-108 cells. IP3R type 1 (IP3R-1) and ankyrin G were not detected. Positive controls of IP3R type 1 and ankyrin G with the mouse cerebellum (CB) are also shown. Numbers with side bullets indicate positions of molecular weight markers (in kDa).
Figure 2
Coupling of Sig-1R, ankyrin B, and IP3R-3 as a trimeric complex. (A) Coimmunoprecipitation (IP) followed by Western blotting of the Sig-1R/ankyrin B/IP3R-3 complex by respective cognate antibodies. (B) Percentages of IP3R-3 and ankyrin B, respectively, coimmunoprecipitated with Sig-1R. IP3R-3 were immunoprecipitated with anti-IP3R-3 antibodies or anti-Sig-1R antibodies. Ankyrin B isoforms were coimmunoprecipitated with either anti-ankyrin B antibodies or anti-Sig-1R antibodies. Immunoblots were digitally scanned and densitometrically quantified by a Macintosh computer-based image analysis program (
image
, National Institutes of Health). Note: Anti-IP3R-3 antibodies and anti-ankyrin B antibodies also immunoprecipitated proteolytic products of IP3R-3 and ankyrin B, respectively (see lanes 1 and 3, from left).
Figure 3
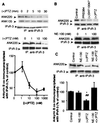
Effects of Sig-1R ligands on the dissociation of ankyrin B (ANK220) from IP3R-3 in NG-108 cells. NG-108 cells were incubated with respective drugs and then lysed for coimmunoprecipitation (IP) studies. (A) Time- and concentration-dependent dissociation of ankyrin B (ANK220) from IP3R-3 by the (+)PTZ treatment. The concentration of (+)PTZ in the top row was 100 nM. For the concentration-dependent curve, immunoblots were digitally scanned and densitometrically analyzed by the National Institutes of Health
image
program. Data were normalized to total immunoprecipitated IP3R-3 proteins and expressed as percentage relative to the level of coimmunoprecipitated ANK220 obtained in the absence of (+)PTZ. Note: Ten-minute treatment time for all concentrations. Data represent mean ± SEM from two to four separate determinations. (B) Effects of selective Sig-1R ligands on ANK220 coupling to IP3R-3. Sig-1R agonists (100 nM, 10 min) decreased the amount of ANK220 coimmunoprecipitated with IP3R-3 without altering the level of IP3R-3. NE-100 (100 nM), a Sig-1R antagonist, did not by itself affect the ANK220 dissociation from IP3R-3. (C) Inhibition of (+)PTZ-induced ANK220 dissociation from IP3R-3 by NE-100. NE-100 (100 nM) was applied 5 min before (+)PTZ (100 nM, 10 min). Data were analyzed as described in_A_ and represent mean ± SEM from six to seven separate determinations. *, P < 0.05 compared with “control”; ##, P < 0.01 compared with “NE-100 + (+)PTZ”.
Figure 4
Regulation of the level of ANK220 in the P3L fraction. (A and B) Distribution of Sig-1R (immunoblotting) and the relative enrichments of subcellular markers for the plasma membrane (Fas), mitochondria (bcl2), Golgi (GM130) (A), rough ER (BiP/GRB78 and RNA), and smooth ER (P450 reductase) (B) in the P1, P2, P3L, and P3H fractions (see Methods). The total density of each marker at the P1, P2, P3L, and P3H fractions from Western blotting were taken as 100%. (C) Top two and bottom two rows: NE-100 blocked the loss of ANK220, but not Sig-1R, caused by the (+)PTZ (100 nM, 10 min) or cocaine treatment (10 μM, 10 min). The IP3R-3 levels remained unaltered under all conditions (middle row). (D) Effects of NE-100 treatment (100 nM, 15 min) by itself on the total levels of Sig-1R and ANK220 (top two rows) and on the portion of ankyrin B coimmunoprecipitated (IP) by anti-Sig-1R antibodies in the P3L fraction (lower row). (E) Effects of (+)PTZ treatment (100 nM, 10 min) on the ANK220 and ANK135 that could be coimmunoprecipitated by anti-Sig-1R antibodies in the whole cell lysate.
Figure 5
Relationship between the degree of dissociation of ANK220 from IP3R-3 induced by Sig-1R ligands and the Sig-1R ligands' abilities to potentiate bradykinin-induced increase in [Ca2 + ]cyt in NG-108 cells. (A) Potentiation of the bradykinin-induced increase in [Ca2+]cyt by (+)PTZ (100 nM, 10 min). (B) Temporal correlation of (+)PTZ (100 nM) in increasing the dissociation of ANK220 from IP3R-3 and in potentiating the [Ca2+]cyt increase induced by bradykinin. The numbers below open circles indicate the treatment time (min) of NG-108 cells with (+)PTZ. (C) Efficacy correlation of Sig-1R ligands in their abilities to cause ANK220 dissociation from IP3R-3 at the 10-min point and in their abilities at the same time point to potentiate bradykinin-induced increases in [Ca2+]cyt. Concentrations of Sig-1R ligands were all 100 nM. The total number of cells examined and the number of determinations (in parentheses) for each test drug are indicated (see_Methods_): NE-100, 23 cells (5); pregnenolone sulfate, 58 cells (7); progesterone, 34 cells (5); PRE084, 91 cells (14); (+)SKF-10047, 36 cells (6); (+)PTZ, 59 cells (8). The value on the_x_ axis represents the mean value from all determinations for each drug. (D) Increase in [3H]IP3 binding to microsomes of NG-108 cells caused by (+)PTZ. After the (+)PTZ treatment (100 nM, 10 min), cells were harvested and P3 fractions prepared and used for [3H]IP3 binding assay. Data represent mean ± SEM of three determinations, each assayed in triplicate. *, P < 0.05.
Figure 6
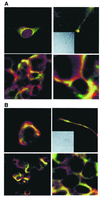
Colocalization of ankyrin, IP3R-3, and Sig-1R in NG-108 cells. (A) Immunocytohistochemical colocalization (yellow) of Sig-1R (green) and ankyrin B (red) in reticular perinuclear areas (Top Left), plasmalemmal regions of cell–cell contact (lower panels in low and high magnifications, respectively), and growth cones of processes in NG-108 cells (Top Right; Inset: Nomarski optical image). (B) Immunocytohistochemical colocalization (yellow) of Sig-1R (green) and IP3R-3 (red) in perinuclear areas (Top Left), plasmalemmal regions of cell–cell contact (lower panels in low and high magnifications, respectively), and growth cones of processes in NG-108 cells (Top Right;Inset: Nomarski optical image).
Figure 7
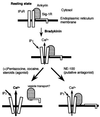
Hypothetical diagram depicting the regulation of ANK220 by Sig-1R and associated ligands. In the presence of a Sig-1R agonist, such as (+)PTZ, cocaine, or certain steroids, the Sig-1R/ankyrin B complex dissociates from IP3R-3 on the ER perhaps as a transport vesicle. As a result, IP3 binding to IP3R-3 increases and the Ca2+ efflux is enhanced. In the presence of the putative Sig-1R antagonist, NE-100, Sig-1R is dissociated from ANK220, which remains coupled to IP3R on the ER.
Similar articles
- Cocaine inhibits store-operated Ca2+ entry in brain microvascular endothelial cells: critical role for sigma-1 receptors.
Brailoiu GC, Deliu E, Console-Bram LM, Soboloff J, Abood ME, Unterwald EM, Brailoiu E. Brailoiu GC, et al. Biochem J. 2016 Jan 1;473(1):1-5. doi: 10.1042/BJ20150934. Epub 2015 Oct 14. Biochem J. 2016. PMID: 26467159 Free PMC article. - Investigation of the role of sigma1-receptors in inositol 1,4,5-trisphosphate dependent calcium signaling in hepatocytes.
Abou-Lovergne A, Collado-Hilly M, Monnet FP, Koukoui O, Prigent S, Coquil JF, Dupont G, Combettes L. Abou-Lovergne A, et al. Cell Calcium. 2011 Jul;50(1):62-72. doi: 10.1016/j.ceca.2011.05.008. Epub 2011 Jun 8. Cell Calcium. 2011. PMID: 21641033 - Role of sigma-1 receptor C-terminal segment in inositol 1,4,5-trisphosphate receptor activation: constitutive enhancement of calcium signaling in MCF-7 tumor cells.
Wu Z, Bowen WD. Wu Z, et al. J Biol Chem. 2008 Oct 17;283(42):28198-215. doi: 10.1074/jbc.M802099200. Epub 2008 Jun 6. J Biol Chem. 2008. PMID: 18539593 Free PMC article. - Role of Sigma-1 Receptor in Cocaine Abuse and Neurodegenerative Disease.
Cai Y, Yang L, Niu F, Liao K, Buch S. Cai Y, et al. Adv Exp Med Biol. 2017;964:163-175. doi: 10.1007/978-3-319-50174-1_12. Adv Exp Med Biol. 2017. PMID: 28315271 Review. - The involvement of the sigma-1 receptor in neurodegeneration and neurorestoration.
Ruscher K, Wieloch T. Ruscher K, et al. J Pharmacol Sci. 2015 Jan;127(1):30-5. doi: 10.1016/j.jphs.2014.11.011. Epub 2014 Dec 9. J Pharmacol Sci. 2015. PMID: 25704015 Review.
Cited by
- HCN1 and HCN2 in Rat DRG neurons: levels in nociceptors and non-nociceptors, NT3-dependence and influence of CFA-induced skin inflammation on HCN2 and NT3 expression.
Acosta C, McMullan S, Djouhri L, Gao L, Watkins R, Berry C, Dempsey K, Lawson SN. Acosta C, et al. PLoS One. 2012;7(12):e50442. doi: 10.1371/journal.pone.0050442. Epub 2012 Dec 7. PLoS One. 2012. PMID: 23236374 Free PMC article. - Sigma1 receptor upregulation after chronic methamphetamine self-administration in rats: a study with yoked controls.
Stefanski R, Justinova Z, Hayashi T, Takebayashi M, Goldberg SR, Su TP. Stefanski R, et al. Psychopharmacology (Berl). 2004 Aug;175(1):68-75. doi: 10.1007/s00213-004-1779-9. Epub 2004 Mar 17. Psychopharmacology (Berl). 2004. PMID: 15029471 - Structural basis for σ1 receptor ligand recognition.
Schmidt HR, Betz RM, Dror RO, Kruse AC. Schmidt HR, et al. Nat Struct Mol Biol. 2018 Oct;25(10):981-987. doi: 10.1038/s41594-018-0137-2. Epub 2018 Oct 5. Nat Struct Mol Biol. 2018. PMID: 30291362 Free PMC article. - Exploring the Association between Schizophrenia and Cardiovascular Diseases: Insights into the Role of Sigma 1 Receptor.
Rafcikova J, Novakova M, Stracina T. Rafcikova J, et al. Physiol Res. 2023 Jul 31;72(Suppl 2):S113-S126. doi: 10.33549/physiolres.935099. Physiol Res. 2023. PMID: 37565416 Free PMC article. Review. - Sigma 1 receptor activation regulates brain-derived neurotrophic factor through NR2A-CaMKIV-TORC1 pathway to rescue the impairment of learning and memory induced by brain ischaemia/reperfusion.
Xu Q, Ji XF, Chi TY, Liu P, Jin G, Gu SL, Zou LB. Xu Q, et al. Psychopharmacology (Berl). 2015 May;232(10):1779-91. doi: 10.1007/s00213-014-3809-6. Epub 2014 Nov 26. Psychopharmacology (Berl). 2015. PMID: 25420607
References
- Bennett V, Stenbuck P J. Nature (London) 1979;280:468–473. - PubMed
- Bennett V. Nature (London) 1979;281:597–599. - PubMed
- Nelson W J, Veshnock P J. Nature (London) 1987;328:533–536. - PubMed
- Srinivasan Y, Elmer L, Davis J, Bennett V, Angelides K. Nature (London) 1988;333:177–180. - PubMed
- De Matteis M A, Morrow J S. Curr Opin Cell Biol. 1998;10:542–549. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous