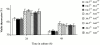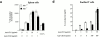c-Jun NH2-terminal kinase (JNK)1 and JNK2 have similar and stage-dependent roles in regulating T cell apoptosis and proliferation - PubMed (original) (raw)
c-Jun NH2-terminal kinase (JNK)1 and JNK2 have similar and stage-dependent roles in regulating T cell apoptosis and proliferation
K Sabapathy et al. J Exp Med. 2001.
Abstract
Apoptotic and mitogenic stimuli activate c-Jun NH2-terminal kinases (JNKs) in T cells. Although T cells express both JNK1 and JNK2 isozymes, the absence of JNK2 alone can result in resistance to anti-CD3-induced thymocyte apoptosis and defective mature T cell proliferation. Similar defects in thymocyte apoptosis and mature T cell proliferation, the latter due to reduced interleukin 2 production, are also caused by JNK1 deficiency. Importantly, T cell function was compromised in Jnk1(+/-)Jnk2(+/-) double heterozygous mice, indicating that JNK1 and JNK2 play similar roles in regulating T cell function. The reduced JNK dose results in defective c-Jun NH2-terminal phosphorylation in thymocytes but not in peripheral T cells, in which nuclear factors of activated T cells (NK-ATs)-DNA binding activity is affected. Thus, JNK1 and JNK2 control similar functions during T cell maturation through differential targeting of distinct substrates.
Figures
Figure 1
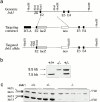
Targeted mutation of the murine Jnk1 gene in ES cells. (a) The structure of Jnk1 genomic DNA encoding a portion of the JNK1 protein kinase domain (top). The targeting vector (middle) replaced parts of exon 2 with a β-galactosidase gene fused in-frame with exon 2 and a Neor cassette driven by the Rous sarcoma virus promoter. The structure of the targeted allele after homologous recombination is depicted (bottom). DT-A, diphtheria toxin A gene used for negative selection. (b) Genotyping of offspring of mice heterozygous for the targeted Jnk1 mutation. Tail DNA was digested with HindIII and analyzed by Southern blot analysis using the probe indicated in panel a. (c) Western blot analysis of JNK protein expression in primary embryonic fibroblasts. Whole cell extracts (100 μg protein) from wild-type (+/+), heterozygous (+/−), and homozygous mutant (−/−) embryonic fibroblasts were separated by SDS-PAGE, transferred to a membrane, and probed with anti-JNK antibodies. The positions of the long (L) and short (S) isoforms of the JNK proteins are indicated.
Figure 2
In vitro susceptibility of Jnk1 − _/_− thymocytes to death caused by direct incubation with anti-CD3 antibody and other apoptotic stimuli. (a–b) Thymocytes from wild-type (⋄) and Jnk1 − _/_− (▪) mice were cultured on 24-well plates coated with anti-CD3ε antibody for (a) 24 h or (b) the indicated time periods in the presence of the indicated concentrations of soluble anti-CD28 antibody, and cell viability was determined. There was no difference in spontaneous (i.e., basal) cell death rates between wild-type and Jnk1 − _/_− thymocytes. (c–h) Cell death was determined after treatment of thymocytes with: (c) various concentrations of dexamethasone; (e) various doses of UVC irradiation; (g) incubation with various concentrations of anti-Fas antibody for 24 h; (d) 10−9 M dexamethasone; (f) 80 J/m2 of UVC irradiation; or (h) 0.05 μg/ml of anti-Fas antibody for the indicated time periods. These results are representative of four independent experiments, each using three pairs of mice. All assays were conducted in triplicates. Standard deviations are indicated by vertical lines.
Figure 4
Jnk1 +/−Jnk2 +/− thymocytes are resistant to anti-CD3 activation–induced cell death. Thymocytes from mice of various genotypes were cultured with anti-CD3ε and anti-CD28 antibodies for 24 h and their viability was determined as described. The results are representative of three independent experiments, each using three matched pairs of mice. All assays were conducted in triplicates. Standard deviations are shown as vertical lines.
Figure 3
Inefficient proliferation and IL-2 production by T cells lacking JNK1. (a–c) Cells from (a) spleen, (b) lymph nodes, or (c) purified splenic T cells, all from 4–8-wk-old Jnk1 +/+ or Jnk1−/− mice, were cultured in the presence of the indicated concentrations of plate-bound anti-CD3ε antibody in the absence or presence of the indicated amounts of anti-CD28 antibody. After 60 h, cells were pulsed for 12 h with 1 μCi [3H]thymidine/well and collected for measurement of [3H]thymidine incorporation into DNA. (d) IL-2 production was measured by ELISA on media collected 24 h after stimulation of purified splenic T cells as described above. (e) Proliferation rates of purified T cells cultured in the presence of 50 U/ml IL-2 plus anti-CD3 and/or anti-CD28 antibodies. Proliferation rates of splenic B cells cultured in medium alone (untreated), or in the presence of either 50 U/ml IL-4 plus 1 μg/ml soluble anti-CD40 or 10 μg/ml LPS. The results are representative of four independent experiments, each using three matched pairs of mice. All assays were conducted in triplicates. Standard deviations are shown as vertical lines.
Figure 3
Inefficient proliferation and IL-2 production by T cells lacking JNK1. (a–c) Cells from (a) spleen, (b) lymph nodes, or (c) purified splenic T cells, all from 4–8-wk-old Jnk1 +/+ or Jnk1−/− mice, were cultured in the presence of the indicated concentrations of plate-bound anti-CD3ε antibody in the absence or presence of the indicated amounts of anti-CD28 antibody. After 60 h, cells were pulsed for 12 h with 1 μCi [3H]thymidine/well and collected for measurement of [3H]thymidine incorporation into DNA. (d) IL-2 production was measured by ELISA on media collected 24 h after stimulation of purified splenic T cells as described above. (e) Proliferation rates of purified T cells cultured in the presence of 50 U/ml IL-2 plus anti-CD3 and/or anti-CD28 antibodies. Proliferation rates of splenic B cells cultured in medium alone (untreated), or in the presence of either 50 U/ml IL-4 plus 1 μg/ml soluble anti-CD40 or 10 μg/ml LPS. The results are representative of four independent experiments, each using three matched pairs of mice. All assays were conducted in triplicates. Standard deviations are shown as vertical lines.
Figure 3
Inefficient proliferation and IL-2 production by T cells lacking JNK1. (a–c) Cells from (a) spleen, (b) lymph nodes, or (c) purified splenic T cells, all from 4–8-wk-old Jnk1 +/+ or Jnk1−/− mice, were cultured in the presence of the indicated concentrations of plate-bound anti-CD3ε antibody in the absence or presence of the indicated amounts of anti-CD28 antibody. After 60 h, cells were pulsed for 12 h with 1 μCi [3H]thymidine/well and collected for measurement of [3H]thymidine incorporation into DNA. (d) IL-2 production was measured by ELISA on media collected 24 h after stimulation of purified splenic T cells as described above. (e) Proliferation rates of purified T cells cultured in the presence of 50 U/ml IL-2 plus anti-CD3 and/or anti-CD28 antibodies. Proliferation rates of splenic B cells cultured in medium alone (untreated), or in the presence of either 50 U/ml IL-4 plus 1 μg/ml soluble anti-CD40 or 10 μg/ml LPS. The results are representative of four independent experiments, each using three matched pairs of mice. All assays were conducted in triplicates. Standard deviations are shown as vertical lines.
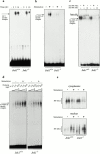
Figure 6
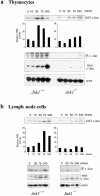
JNK activity and c-Jun status in Jnk1 − _/_− cells. (a) Thymocytes and (b) lymph node cells from wild-type and Jnk1 − _/_− mice were stimulated for the indicated times with 10 μg/ml anti-CD3 plus 0.1 μg/ml anti-CD28 antibodies, and JNK activity was determined in whole cell extracts, using solid-state kinase assay and GST–c-Jun (1-79) as a substrate. The same extracts were analyzed for their content of phospho-c-Jun (S63), total c-Jun, and actin by Western blot analysis.
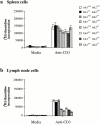
Figure 5
Impaired proliferation of Jnk1+ / −Jnk2+/− T cells. (a) Spleen cells and (b) lymph node cells were cultured with 1 μg/ml of anti-CD3 antibody and proliferation rates were determined as described above. The results are representative of three independent experiments, each using three matched pairs of mice. All assays were conducted in triplicates. Standard deviations are shown as vertical lines.
Figure 7
AP-1– and NF-AT–DNA-binding activity in JNK1-deficient T cells. (a) AP-1–DNA binding activity was measured using 2.5 μg nuclear extracts from untreated or 10 μg/ml anti-CD3 and 0.1 μg/ml anti-CD28–stimulated lymph node cells for the indicated period of time. The proteins were incubated with an AP-1 binding site oligonucleotide probe, and the specific protein–DNA complex is indicated. (b–d) NF-AT–DNA binding activity from untreated (−) or anti-CD3– and anti-CD28–stimulated (3 h; +) lymph node cells of wild-type and Jnk1 − _/_− mice was examined using oligonucleotide probes corresponding to either the low affinity NF-AT binding site from the IL-2 promoter (b and c) or the high affinity NF-AT binding site from the IL-4 promoter (d). 2.5 μg of nuclear extracts was used in b. In the supershift experiments, 2.5 μg of wild-type extracts and 12.5 μg of Jnk1 − _/_− extracts were used and the specific complex was supershifted by both anti–NF-ATc1 and anti–NF-ATc2 antibodies (c). Increasing amounts of extracts were used in d as indicated. The specific protein–DNA complex is indicated. (e) Cytoplasmic and nuclear extracts from unstimulated (−) and stimulated (+) wild-type and Jnk1 − _/_− lymph node T cells were prepared as described in b–d, and NF-ATc1 protein was detected by Western blot analysis.
Similar articles
- Jun N-terminal kinase 2 modulates thymocyte apoptosis and T cell activation through c-Jun and nuclear factor of activated T cell (NF-AT).
Behrens A, Sabapathy K, Graef I, Cleary M, Crabtree GR, Wagner EF. Behrens A, et al. Proc Natl Acad Sci U S A. 2001 Feb 13;98(4):1769-74. doi: 10.1073/pnas.98.4.1769. Proc Natl Acad Sci U S A. 2001. PMID: 11172026 Free PMC article. - JNK2 is required for efficient T-cell activation and apoptosis but not for normal lymphocyte development.
Sabapathy K, Hu Y, Kallunki T, Schreiber M, David JP, Jochum W, Wagner EF, Karin M. Sabapathy K, et al. Curr Biol. 1999 Feb 11;9(3):116-25. doi: 10.1016/s0960-9822(99)80065-7. Curr Biol. 1999. PMID: 10021384 - Distinct roles for JNK1 and JNK2 in regulating JNK activity and c-Jun-dependent cell proliferation.
Sabapathy K, Hochedlinger K, Nam SY, Bauer A, Karin M, Wagner EF. Sabapathy K, et al. Mol Cell. 2004 Sep 10;15(5):713-25. doi: 10.1016/j.molcel.2004.08.028. Mol Cell. 2004. PMID: 15350216 - JNK2: a negative regulator of cellular proliferation.
Sabapathy K, Wagner EF. Sabapathy K, et al. Cell Cycle. 2004 Dec;3(12):1520-3. doi: 10.4161/cc.3.12.1315. Epub 2004 Dec 18. Cell Cycle. 2004. PMID: 15611655 Review. - From JNK to pay dirt: jun kinases, their biochemistry, physiology and clinical importance.
Karin M, Gallagher E. Karin M, et al. IUBMB Life. 2005 Apr-May;57(4-5):283-95. doi: 10.1080/15216540500097111. IUBMB Life. 2005. PMID: 16036612 Review.
Cited by
- Fam49b dampens TCR signal strength to regulate survival of positively selected thymocytes and peripheral T cells.
Park CS, Guan J, Rhee P, Gonzalez F, Lee HS, Park JH, Coscoy L, Robey EA, Shastri N, Sadegh-Nasseri S. Park CS, et al. Elife. 2024 Aug 19;13:e76940. doi: 10.7554/eLife.76940. Elife. 2024. PMID: 39158947 Free PMC article. - A stepwise and digital pattern of RSK phosphorylation determines the outcome of thymic selection.
Funasaki S, Hatano A, Nakatsumi H, Koga D, Sugahara O, Yumimoto K, Baba M, Matsumoto M, Nakayama KI. Funasaki S, et al. iScience. 2023 Aug 9;26(9):107552. doi: 10.1016/j.isci.2023.107552. eCollection 2023 Sep 15. iScience. 2023. PMID: 37646020 Free PMC article. - Author Correction: A central role for JNK in obesity and insulin resistance.
Hirosumi J, Tuncman G, Chang L, Görgün CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. Hirosumi J, et al. Nature. 2023 Jul;619(7968):E25. doi: 10.1038/s41586-023-06285-0. Nature. 2023. PMID: 37328696 No abstract available. - A systems and computational biology perspective on advancing CAR therapy.
Tserunyan V, Finley SD. Tserunyan V, et al. Semin Cancer Biol. 2023 Sep;94:34-49. doi: 10.1016/j.semcancer.2023.05.009. Epub 2023 May 30. Semin Cancer Biol. 2023. PMID: 37263529 Free PMC article. Review. - DExD/H-box helicase 9 intrinsically controls CD8+ T cell-mediated antiviral response through noncanonical mechanisms.
Jiao A, Sun C, Wang X, Lei L, Liu H, Li W, Yang X, Zheng H, Ding R, Zhu K, Su Y, Zhang C, Zhang L, Zhang B. Jiao A, et al. Sci Adv. 2022 Feb 11;8(6):eabk2691. doi: 10.1126/sciadv.abk2691. Epub 2022 Feb 9. Sci Adv. 2022. PMID: 35138904 Free PMC article.
References
- Crabtree G.R. Contingent genetic regulatory events in T lymphocyte activation. Science. 1989;243:355–361. - PubMed
- Su B., Jacinto E., Hibi M., Kallunki T., Karin M., Ben-Neriah Y. JNK is involved in signal integration during costimulation of T lymphocytes. Cell. 1994;77:727–736. - PubMed
- Jacinto E., Werlen G., Karin M. Cooperation between Syk and Rac1 leads to synergistic JNK activation in T lymphocytes. Immunity. 1998;8:31–41. - PubMed
- Healy J.I., Dolmetsch R.E., Timmerman L.A., Cyster J.G., Thomas M.L., Crabtree G.R., Lewis R.S., Goodnow C.C. Different nuclear signals are activated by the B cell receptor during positive versus negative signaling. Immunity. 1997;6:419–428. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous