Adaptive plasticity in tachykinin and tachykinin receptor expression after focal cerebral ischemia is differentially linked to gabaergic and glutamatergic cerebrocortical circuits and cerebrovenular endothelium - PubMed (original) (raw)
Adaptive plasticity in tachykinin and tachykinin receptor expression after focal cerebral ischemia is differentially linked to gabaergic and glutamatergic cerebrocortical circuits and cerebrovenular endothelium
R Stumm et al. J Neurosci. 2001.
Abstract
To test the hypothesis of an involvement of tachykinins in destabilization and hyperexcitation of neuronal circuits, gliosis, and neuroinflammation during cerebral ischemia, we investigated cell-specific expressional changes of the genes encoding substance P (SP), neurokinin B (NKB), and the tachykinin/neurokinin receptors (NK1, NK2, and NK3) after middle cerebral artery occlusion (MCAO) in the rat. Our analysis by quantitative in situ hybridization, immunohistochemistry, and confocal microscopy was concentrated on cerebrocortical areas that survive primary infarction but undergo secondary damage. Here, SP-encoding preprotachykinin-A and NK1 mRNA levels and SP-like immunoreactivity were transiently increased in GABAergic interneurons at 2 d after MCAO. Coincidently, MCAO caused a marked expression of SP and NK1 in a subpopulation of glutamatergic pyramidal cells, and in some neurons SP and NK1 mRNAs were coinduced. Elevated levels of the NKB-encoding preprotachykinin-B mRNA and of NKB-like immunoreactivity at 2 and 7 d after MCAO were confined to GABAergic interneurons. In parallel, the expression of NK3 was markedly downregulated in pyramidal neurons. MCAO caused transient NK1 expression in activated cerebrovenular endothelium within and adjacent to the infarct. NK1 expression was absent from activated astroglia or microglia. The differential ischemia-induced plasticity of the tachykinin system in distinct inhibitory and excitatory cerebrocortical circuits suggests that it may be involved in the balance of endogenous neuroprotection and neurotoxicity by enhancing GABAergic inhibitory circuits or by facilitating glutamate-mediated hyperexcitability. The transient induction of NK1 in cerebrovenular endothelium may contribute to ischemia-induced edema and leukocyte diapedesis. Brain tachykinin receptors are proposed as potential drug targets in stroke.
Figures
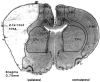
Fig. 1.
Low-power micrograph of a cresyl violet-stained 20-μm-thick frozen coronal section through a rat forebrain 2 d after MCAO. The pale infarcted area is clearly delineated and includes the forelimb (FL) and parietal (Par) areas of the cortex and large parts of the insular (I) and piriform (Pir) cortex. The caudate-putamen (CPu) is only infarcted in its exterior part. The cingulate (Cg) and frontal (Fr) cortex is not infarcted. Note the swelling of the infarcted hemisphere as compared with the contralateral hemisphere.
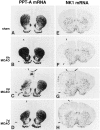
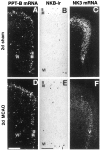
Fig. 2.
Spatiotemporal patterns of changes in cerebral PPT-A (A–D) and NK1 mRNA (E–H) expression after MCAO. Low-power micrographs of x-ray autoradiograms after _in situ_hybridization of coronal sections through the forebrain are shown. The lateral borders of the infarcted area at each stage after MCAO are marked by arrowheads. For identification of the different brain regions see Figure 1. A, E, PPT-A mRNA and NK1 mRNA levels of expression, respectively, are low in the ipsilateral and contralateral cortex of a rat 2 d after sham operation. B–D, Note a marked increase in PPT-A mRNA levels (C, arrows) in noninfarcted ipsilateral cingulate, frontal, insular, and piriform cortical areas at 2 d after MCAO. PPT-A mRNA is completely lost in the infarcted cortex (B–D). In the dorsal caudate-putamen at 6 hr after MCAO (B), there is a marked increase in PPT-A mRNA levels on the ipsilateral side and a less marked increase on the contralateral side as compared with levels in A. [Note that the contralateral increase seen in this animal was not seen in all animals of this experimental group; in the dorsal caudate-putamen, there was no statistically significant difference between contralateral and sham PPT-A mRNA levels (see Table 1).] At 2 d after MCAO (C), PPT-A mRNA is lost in the infarcted lateral part of the caudate-putamen and decreased in the noninfarcted ipsilateral caudate-putamen as compared with the sham animal (A). At 7 d after MCAO (D), PPT-A mRNA levels in the noninfarcted caudate-putamen return to the levels seen after sham treatment (A). F–H, At 2 d after MCAO (G) but not at 6 hr (F) or 7 d (H) after MCAO, NK1 mRNA expression is induced in a distinct band (G,arrow) of the ipsilateral cingulate and frontal cortex as compared with a sham-operated animal (E) (for high magnification see Fig. 3). NK1 mRNA is present in the meningeal circumference of the infarcted area at 2 d (G) and at 7 d (H) after MCAO but is not present at 6 hr after MCAO (F) or in the sham-treated rat (E). Note the presence of some NK1 mRNA expression in the infarcted area at all stages after MCAO (for high magnification see Fig. 5). Sham-operated animals 2 d after surgery (A, E) are representative of the sham-operated animals 6 hr and 7 d after surgery. Exposure times: A–D, 24 hr; E–H, 72 hr.
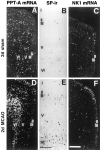
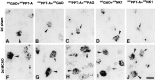
Fig. 3.
High-power dark- and bright-field micrographs of ipsilateral cingulate and frontal cortex demonstrating changes in the cellular localization and expression levels of PPT-A and NK1 mRNA and SP-like immunoreactivity (SP-ir) 2 d after MCAO. A, D, Note an increase in PPT-A mRNA levels per neuron and in the number of PPT-A mRNA-expressing neurons in laminae II–VI (D) as compared with those in the sham animal (A). B, E, There is a marked increase in the number of SP-immunoreactive neurons after MCAO (E) as compared with that in the sham animal (B). C, F, NK1 mRNA levels after MCAO are increased in laminae II–III (F) as compared with that in the sham animal (C). Cortical laminae are indicated by Roman numerals. Exposure times: A, D, 9 d; B, E, no exposure; C, F, 42 d. Scale bars:A, D, C, F, 500 μm; B, E, 150 μm.
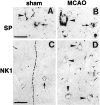
Fig. 4.
High-power immunocytochemistry for SP and NK1 receptor in the frontocingulate cortex at 2 d after sham treatment (A, C) and 2 d after MCAO (B, D).A, Absence of SP-ir from the perikaryon of a lamina V pyramidal neuron heavily invested by SP-like-immunoreactive terminals (arrowhead) and presence in a small nonpyramidal neuron (arrow). B, Presence of strong SP-ir in the perikaryon of a lamina V pyramidal neuron (arrowhead) and less strong SP-ir in the perikaryon of a small nonpyramidal neuron (arrow). Note the SP-like-immunoreactive terminals around a small SP-negative nonpyramidal neuron below the SP-positive pyramidal neuron.C, Presence of weak NK1-ir in a small nonpyramidal neuron (arrow) and in a trespassing fiber strand.D, Presence of moderately intense NK1-ir in several perikarya of pyramidal neurons of laminae II–III (arrowheads) and in some terminals. Scale bars, 25 μm.
Fig. 5.
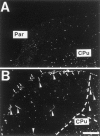
Comparision of NK1 expression in the parietal cortex (Par) of a rat 2 d after sham operation (A) and a rat 2 d after MCAO (B). Dark-field micrographs of coronal sections after in situ hybridization demonstrate the expression of NK1 mRNA in the leptomeninx at the circumference of the infarct (B, arrows) but not in the leptomeninx of a rat 2 d after sham operation (A). Note the NK1 mRNA induction in vascular structures in the infarcted area and in juxtaposition to the infarct (B,arrowheads). The dotted line marks the border of the infarcted area. Exposure time, 42 d. Scale bar, 1 mm. CPu, Caudate-putamen.
Fig. 6.
High-power in situ hybridization and immunohistochemical analysis of the cellular localization of NK1 expression in the parietal cortex 2 d after MCAO as compared with sham operation. B, After MCAO, NK1 mRNA is present in endothelial cells (arrowheads), in an intraluminal leukocyte (bold arrow) adhering to the endothelium, and in a paravascular cell (arrow) of a blood vessel in the leptomeninx covering the infarcted area. A, In contrast, note the absence of any NK1 mRNA hybridization signal related to the wall of a meningeal blood vessel of a sham-operated rat.D, NK1-ir is strongly induced in the endothelium (arrowhead) of a meningeal blood vessel that extends into lamina I of the noninfarcted cortex immediately adjacent to the infarcted cortex and is also present in some varicose fibers.C, In a sham-operated rat, NK1-ir is absent from the endothelium of a meningeal blood vessel extending into lamina II of the parietal cortex, whereas a paravascular neuron and a trespassing varicose fiber are NK1 immunopositive. E, F, Adjacent sections alternately immunostained for NK1 (E) and CD62-P, a marker of activated venular endothelium (F), reveal the presence of NK1 in the endothelium of most CD62-P-positive cerebral venules (single arrowheads). One double-labeled venule (double arrowheads) is shown at high power as an_inset_ in E and F. Small blood vessels immunonegative for both NK1 and CD62-P are marked by_long arrows_ (E, F). The_short arrow_ in E marks NK1-ir related to neurons and fibers in the adjacent noninfarcted caudate-putamen.H, At 2 d after MCAO, note the accumulation of SP-like-immunoreactive fibers and terminals (arrowheads) in the perivascular space of a small blood vessel (asterisk) located in the infarcted cortex.G, At 2 d after sham treatment, note the absence of SP-like-immunoreactive fibers from the perivascular space (arrowhead) of a cortical blood vessel (asterisk). Exposure times: A, B, 42 d. Scale bars: A, D, 33.3 μm;B, 20 μm; C, E–H, 100 μm;insets, E, F, 50 μm.
Fig. 7.
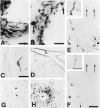
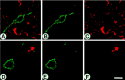
Absence of NK1-ir from activated astrocytes and microglial cells. False-color micrographs are shown of confocal images from double immunofluorescence for NK1 (green;A, B, D, E) and GFAP (red; A, C) and C1q (red; D, F) in the frontal cortex ipsilateral to the lesion 2 d after MCAO. NK1-like-immunoreactive neurons (A, B, D, E) exhibit a strong somatodendritic labeling; the heavy cytosolic NK1-ir is evidence of NK1 internalization. GFAP-like-immunoreactive profiles (A, C) of activated astrocytes and C1q-like-immunoreactive profiles (D, F) of an activated microglial cell are clearly distinct from NK1-like immunolabeling. Scale bar, 20 μm.
Fig. 8.
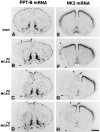
Spatiotemporal patterns of changes in cerebral PPT-B and NK3 mRNA expression after MCAO. Low-power micrographs of x-ray autoradiograms after in situ hybridization of coronal sections through the forebrain are shown. For identification of brain areas see nomenclature in Figure 1. The lateral borders of the infarcted area at each stage after MCAO are marked by_arrowheads_. Note that PPT-B and NK3 mRNAs become completely depleted from the infarcted area (B–D, F–H). A–D, PPT-B mRNA levels in the noninfarcted ipsilateral cingulate and frontal cortex (B–D, arrows) are increased 2 d (C) and 7 d (D) after MCAO as compared with the levels in an animal 2 d after sham operation (A). In the ipsilateral caudate-putamen, PPT-B mRNA levels are increased at 2 and 7 d after MCAO (C, D) as compared with the levels in the sham-treated animal (A). E–H, Note the dramatic decrease in NK3 mRNA levels in the ipsilateral cingulate and frontal cortex 2 d after MCAO (G, arrow) as compared with the levels in an animal 2 d after sham operation (E) (for high magnification see Fig.9_F_). At 7 d after MCAO (H), NK3 mRNA levels appear only marginally decreased in the ipsilateral cingulate and frontal cortex (H, arrow) as compared with the levels in the sham-operated animal (E). Sham-operated animals 2 d after surgery (A, E) are representative of sham animals 6 hr and 7 d after surgery (data not shown). Exposure times: A–D, 36 hr; E–H, 48 hr. Scale bar, 2.5 mm.
Fig. 9.
High-power dark- and bright-field micrographs of ipsilateral cingulate and frontal cortex demonstrating cellular localization and changes in the expression levels of PPT-B mRNA (A, D), NKB-ir (B, E), and NK3 mRNA (C, F) 2 d after MCAO as compared with the levels after a sham operation. A, D, After MCAO, there is a marked increase in PPT-B mRNA levels per neuron (D) and a small increase in the number of PPT-B mRNA-expressing neurons in laminae II–III and VI (D) as compared with those in a sham animal (A). B, E, Note the increase in the levels of NKB-ir per cell and the small increase in neurons showing NKB-ir in laminae II–III and VI after MCAO (E) as compared with the sham operation (B).C, F, There is a dramatic reduction of NK3 mRNA levels in virtually all NK3 mRNA-expressing neurons in lamina V after MCAO (F) as compared with the sham operation (C). Cortical laminae are indicated by_Roman numerals_. Exposure times: A, D, 16 d; C, F, 21 d. Scale bars: A, C, D, F, 500 μm; B, E, 150 μm.
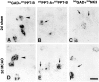
Fig. 10.
High-resolution double in situ_hybridization analysis of the influence of MCAO on PPT-A and NK1 mRNA cophenotypes in cerebrocortical neurons by the use of 35S- and digoxigenin-labeled riboprobes. Digoxigenin labeling (DIG) is recognized as a black reaction product, and 35S labeling (35S) is seen as_grains. A, B, F, G, After both sham operation (A, B) and MCAO (F, G), PPT-A mRNA and GAD mRNA coexist in a neuronal subpopulation (arrowheads). After MCAO, PPT-A mRNA is induced in a subpopulation of GAD mRNA-negative neurons (F, G, arrows). C, H, In a sham-operated rat, PPT-A mRNA is present in a PAG mRNA-negative neuron (C,arrow) but not in PAG mRNA-positive neurons (C, asterisks). After MCAO, PPT-A mRNA is induced in a PAG mRNA-positive neuron (H,arrowheads) in addition to being expressed in a PAG mRNA-negative neuron (H, arrow). PAG mRNA-positive neurons that are PPT-A mRNA negative are labeled by an_asterisk_ in H. D, I, In a sham-operated animal, NK1 mRNA expression is restricted to a subpopulation of GAD mRNA-positive neurons (D,arrowhead). After MCAO, NK1 mRNA is induced in a GAD mRNA-negative neuron (I, arrow) and is also expressed in a GAD mRNA-positive neuron (I,arrowhead). E, J, In a sham-operated animal, NK1 mRNA is confined to a PPT-A mRNA-negative neuron (E, asterisk). After MCAO (J), NK1 mRNA is induced in PPT-A mRNA-positive neurons (J, arrowheads); in addition NK1 mRNA is present in a PPT mRNA-negative neuron (J,asterisk). Note the PPT-A mRNA-positive neurons with no signal for NK1 mRNA (E, J, arrows). Exposure times: A–C, F–H, 14 d; D, E, I, J, 21 d. Scale bar, 25 μm.
Fig. 11.
High-resolution double _in situ_hybridization analysis of the influence of MCAO on PPT-B and NK3 mRNA cophenotypes in cerebrocortical neurons by the use of 35S (35S)- and digoxigenin (DIG)-labeled riboprobes. A, D, After both sham operation (A) and MCAO (D), PPT-B mRNA is confined to a subpopulation of GAD mRNA-positive neurons (arrowheads). B, E, PPT-A (arrows) and PPT-B (asterisks) mRNA expression occurs in different neuronal populations, both after sham treatment and after MCAO. C, F, NK3 mRNA expression occurs in GAD mRNA-negative neurons of a sham-operated rat (C, arrows). After MCAO, NK3 mRNA is decreased in a GAD mRNA-negative neuron (F,arrow). Exposure time, 14 d. Scale bar, 25 μm.
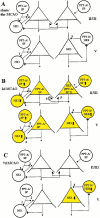
Fig. 12.
Schematic diagrams summarizing presumed cerebrocortical tachykininergic circuits and their ischemia-induced alterations in relation to inhibitory GABAergic and excitatory glutamatergic pathways. GABAergic interneurons and glutamatergic pyramidal cells are depicted as large circles and_triangles_, respectively. Inhibitory and excitatory terminals are symbolized as small filled circles and_triangles_, respectively. Cortical laminae are symbolized by Roman numerals. Symbols and principles of cortical circuits were adopted from Somogyi et al. (1998).A, Tachykininergic circuits at the early stage (6 hr) after focal ischemia that are not altered and correspond to control conditions. SP synthesis is restricted to NK1-negative GABAergic interneurons. SPergic/GABAergic interneurons project to non-SPergic/GABAergic interneurons and pyramidal cells (1,2). SP released from these GABAergic interneurons is likely to act on somatodendritic NK1 receptors (1) and, hypothetically, on NK1 receptors presynaptic (2) to pyramidal cells. This may result in increased release of GABA and, thus, facilitation of GABAergic inhibition of pyramidal neurons. NKB is synthesized in SP-negative GABAergic interneurons, and NK3 is synthesized in pyramidal cells of lamina V. NKB is proposed to be an excitatory cotransmitter in local GABAergic circuits with synaptic input to NK3-expressing pyramidal cells (3).B, Tachykininergic circuit alterations at 2 d after ischemia and their functional implications. SP expression is increased in GABAergic interneurons. Enhanced neurotransmission of SP at their projection sites (1 + 2) may result in a reinforcement of the presumed NK1-mediated facilitation of GABAergic inhibition of pyramidal neurons. The increased synthesis of NK1 in GABAergic interneurons may compensate for presumed NK1 receptor desensitization. SP is de novo synthesized in pyramidal cells of laminae II–V, and NK1 synthesis is induced in pyramidal cells of laminae II–III with a possible NK1 and SP coinduction. Thus, SP released as an excitatory cotransmitter from glutamatergic pyramidal neurons may have dual functions, both to contribute to the activation of the GABAergic inhibitory pathway (4) and to stimulate the glutamatergic excitatory pathway via activation of pyramidal NK1 heteroreceptors (5) or autoreceptors (6). Furthermore, the _de novo_-expressed NK1 receptors of pyramidal glutamatergic neurons are likely targets of SP released from GABAergic interneurons (7). NKB synthesis in SP-negative GABAergic interneurons is enhanced and possibly paralleled by increased NKB release at NK3-positive pyramidal cells (3). This is presumed to add to the activation of the glutamatergic excitatory pathway.C, Tachykininergic circuitry alterations and partial normalizations at 7 d after ischemia. Increases in SP and NK1 expression seen at 2 d in both GABAergic and glutamatergic pathways are no longer present (A). In contrast, the synthesis of NKB in GABAergic interneurons remains increased at approximately the same level seen after 2 d. The decrease of NK3 seen in pyramidal neurons at 2 d is almost normalized to levels seen in controls. Thus, functional alterations at 7 d after ischemia are limited to the NKB/NK3-mediated influence on excitatory glutamatergic neurotransmission of pyramidal neurons.
Similar articles
- A role for tachykinins in female mouse and rat reproductive function.
Pintado CO, Pinto FM, Pennefather JN, Hidalgo A, Baamonde A, Sanchez T, Candenas ML. Pintado CO, et al. Biol Reprod. 2003 Sep;69(3):940-6. doi: 10.1095/biolreprod.103.017111. Epub 2003 May 28. Biol Reprod. 2003. PMID: 12773411 - Characterization of tachykinin receptors in the uterus of the oestrogen-primed rat.
Magraner J, Pinto FM, Anselmi E, Hernandez M, Perez-Afonso R, Martín JD, Advenier C, Candenas ML. Magraner J, et al. Br J Pharmacol. 1998 Jan;123(2):259-68. doi: 10.1038/sj.bjp.0701613. Br J Pharmacol. 1998. PMID: 9489614 Free PMC article. - Gastric ulcer induced changes in substance P and Nk1, Nk2, Nk3 receptors expression in different stomach localizations with regard to intrinsic neuronal system.
Zalecki M. Zalecki M. Histochem Cell Biol. 2019 Jan;151(1):29-42. doi: 10.1007/s00418-018-1715-4. Epub 2018 Aug 28. Histochem Cell Biol. 2019. PMID: 30155561 Free PMC article. - Tachykinins in the gut. Part I. Expression, release and motor function.
Holzer P, Holzer-Petsche U. Holzer P, et al. Pharmacol Ther. 1997;73(3):173-217. doi: 10.1016/s0163-7258(96)00195-7. Pharmacol Ther. 1997. PMID: 9175155 Review. - Molecular recognition of tachykinin receptor selective agonists: insights from structural studies.
Ganjiwale A, Cowsik SM. Ganjiwale A, et al. Mini Rev Med Chem. 2013 Dec;13(14):2036-46. doi: 10.2174/13895575113139990079. Mini Rev Med Chem. 2013. PMID: 23937231 Review.
Cited by
- CXCR4 regulates interneuron migration in the developing neocortex.
Stumm RK, Zhou C, Ara T, Lazarini F, Dubois-Dalcq M, Nagasawa T, Höllt V, Schulz S. Stumm RK, et al. J Neurosci. 2003 Jun 15;23(12):5123-30. doi: 10.1523/JNEUROSCI.23-12-05123.2003. J Neurosci. 2003. PMID: 12832536 Free PMC article. - Neuroprotection by endogenous and exogenous PACAP following stroke.
Chen Y, Samal B, Hamelink CR, Xiang CC, Chen Y, Chen M, Vaudry D, Brownstein MJ, Hallenbeck JM, Eiden LE. Chen Y, et al. Regul Pept. 2006 Nov 15;137(1-2):4-19. doi: 10.1016/j.regpep.2006.06.016. Epub 2006 Oct 4. Regul Pept. 2006. PMID: 17027094 Free PMC article. - CXC chemokine receptor 7 (CXCR7) regulates CXCR4 protein expression and capillary tuft development in mouse kidney.
Haege S, Einer C, Thiele S, Mueller W, Nietzsche S, Lupp A, Mackay F, Schulz S, Stumm R. Haege S, et al. PLoS One. 2012;7(8):e42814. doi: 10.1371/journal.pone.0042814. Epub 2012 Aug 6. PLoS One. 2012. PMID: 22880115 Free PMC article. - NK1-r Antagonist Treatment Comparable to Decompressive Craniectomy in Reducing Intracranial Pressure Following Stroke.
Sorby-Adams AJ, Leonard AV, Hoving JW, Yassi N, Vink R, Wells AJ, Turner RJ. Sorby-Adams AJ, et al. Front Neurosci. 2019 Jul 5;13:681. doi: 10.3389/fnins.2019.00681. eCollection 2019. Front Neurosci. 2019. PMID: 31333402 Free PMC article. - PET imaging of neurokinin-1 receptors with [(18)F]SPA-RQ in human subjects: assessment of reference tissue models and their test-retest reproducibility.
Yasuno F, Sanabria SM, Burns D, Hargreaves RJ, Ghose S, Ichise M, Chin FT, Morse CL, Pike VW, Innis RB. Yasuno F, et al. Synapse. 2007 Apr;61(4):242-51. doi: 10.1002/syn.20361. Synapse. 2007. PMID: 17230546 Free PMC article.
References
- Angerer LM, Cox KH, Angerer RC. Demonstration of tissue-specific gene expression by in situ hybridization. Methods Enzymol. 1987;152:649–661. - PubMed
- Baluk P, Bowden JJ, Lefevre PM, McDonald DM. Upregulation of substance P receptors in angiogenesis associated with chronic airway inflammation in rats. Am J Physiol. 1997;273:L565–L571. - PubMed
- Belayev L, Busto R, Zhao W, Ginsberg MD. Quantitative evaluation of blood-brain barrier permeability following middle cerebral artery occlusion in rats. Brain Res. 1996;739:88–96. - PubMed
- Blanchet F, Gauchy C, Perez S, Soubrie P, Glowinski J, Kemel ML. Distinct modifications by neurokinin1 (SR140333) and neurokinin2 (SR48968) tachykinin receptor antagonists of the N-methyl-d-aspartate-evoked release of acetylcholine in striosomes and matrix of the rat striatum. Neuroscience. 1998;85:1025–1036. - PubMed
- Bonner TI, Affolter HU, Young AC, Young WD. A cDNA encoding the precursor of the rat neuropeptide, neurokinin B. Brain Res. 1987;388:243–249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources