The AN2 protein is a novel marker for the Schwann cell lineage expressed by immature and nonmyelinating Schwann cells - PubMed (original) (raw)
The AN2 protein is a novel marker for the Schwann cell lineage expressed by immature and nonmyelinating Schwann cells
S Schneider et al. J Neurosci. 2001.
Abstract
The expression of the 330 kDa AN2 glycoprotein was studied in the rodent peripheral nervous system. AN2 is expressed by immature Schwann cells in vitro and in vivo and downregulated as the cells upregulate myelin genes. A subpopulation of nonmyelinating Schwann cells in the adult sciatic nerve retains expression of AN2. In rat sciatic nerve crushes, where Schwann cell numbers increase after initial axonal loss and markers of immature Schwann cells show an upregulation, no increased expression of AN2 was observed. In contrast, AN2 expression was upregulated in nerves from peripheral myelin protein-22-transgenic rats, where immature Schwann cells expand without axonal loss. Furthermore, coculture with neurons upregulated AN2 expression on Schwann cells in vitro. Polyclonal antibodies against AN2 inhibited the migration of an immortalized Schwann cell clone in an in vitro migration assay, and the purified AN2 protein was shown to be neither inhibitory nor permissive for outgrowing dorsal root ganglion neurites. AN2 is thus a novel marker for the Schwann cell lineage. Matrix-assisted laser desorption/ionization time-of-flight mass spectrometry analysis of purified AN2 from early postnatal mouse brain demonstrated that AN2 is the murine homolog of the rat NG2 proteoglycan.
Figures
Fig. 1.
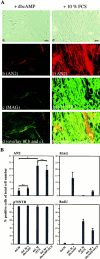
The expression of the AN2 protein by Schwann cells is upregulated by serum factors. A, Primary SCs were cultured for 2 d in chemically defined medium with addition of 1 m
m
dbcAMP (a–d) or 10% FCS (e–h) and subsequently stained with AN2 polyclonal (b) and monoclonal (f) antibodies, MAG monoclonal antibodies (c), and p75NTR
Fig. 2.
The expression of the AN2 protein by Schwann cells is upregulated in coculture with DRG neurons. A, DRG neurons from P0 mice were cultured for 5 d in BME with 10% HS, 200 ng/ml NGF, and 50 m
m
AraC. Purified primary SCs were then added, and culturing continued for 12 d in def. medium with 1% HS and 200 ng/ml NGF. The cocultures were stained with AN2 polyclonal antibodies (b) and L1 monoclonal antibodies
Fig. 3.
The AN2 protein is expressed by immature and nonmyelinating Schwann cells in vivo. Left, Middle, Longitudinal cryosections of P8 (a–d) and adult (e–h) mouse sciatic nerve (SN) were stained with AN2 monoclonal (b) and polyclonal (f) antibodies, p75NTR polyclonal antibodies (c), and L1 monoclonal antibodies (g). a_and e are the corresponding phase-contrast pictures.d shows the overlay of b and_c, and h shows the overlay of_f_ and g. The arrowhead in_h_ indicates an AN2 and L1 double-labeled Schwann cell, and the arrow in f points to AN2-positive cells of the perineurium. Right, A longitudinal section of adult rat SN (i–l) was stained with AN2 polyclonal antibodies (j) and MAG monoclonal antibodies (k). i shows the corresponding phase-contrast picture, and l shows the overlay of j and k. Single optical sections were analyzed by use of a confocal microscope. Scale bars:a–d, e–h, i–l, 10 μm.
Fig. 4.
Expression of the AN2 protein during postnatal development of the PNS. A, Octylglucoside (60 m
m
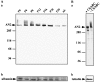
) lysates (each containing 50 μg of protein) from mouse sciatic nerves of different developmental stages were subjected to electrophoretic separation and Western blotting. a, The blot was then incubated with AN2 monoclonal antibodies and developed with the ECL system (Amersham-Buchler). b, The membrane was stained with Poinceau S before antibody incubation, and the albumin band at 66 kDa is shown to verify that equal amounts of protein were loaded per lane. B, a, Forty micrograms of total protein from P8 mouse sciatic nerve lysate as described in_Aa_ were incubated with (+) or without (−) 10 mU of chondroitinase ABC (ChABC) for 5 hr at 37°C before gel electrophoresis and Western blotting with AN2 polyclonal antibodies.b, The blot was reprobed with α-tubulin antibodies to confirm that equal amounts of protein had been loaded in each_lane_. ad., Adult. Molecular weight markers are shown on the left.
Fig. 5.
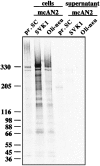
Synthesis and posttranslational modification of the AN2 protein in primary Schwann cells and the Schwann cell clone SVK1 compared with that of the oligodendroglial cell line Oli-neu. Immunoprecipitation with monoclonal AN2 antibodies (mcAN2) from cell lysates and supernatants of primary SCs (pr. SC), the SC clone SVK1, and the oligodendroglial cell line Oli-neu after radiolabeling is shown.
Fig. 6.
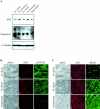
The expression level of the AN2 protein is not changed after sciatic nerve crush. A, Lysates of different sciatic nerve probes from operated and unoperated rats were separated by gel electrophoresis, blotted, and incubated with polyclonal antibodies against AN2. After stripping, the same blot was incubated with polyclonal antibodies against α-tubulin and polyclonal antibodies against tenascin-C. Equal amounts of protein (50 μg) were loaded per lane. B, Longitudinal cryosections of distal nerve stumps 1 week (a–c) and 3 weeks (d–f) after nerve crush and from unoperated littermates (g–i) were stained with AN2 polyclonal antibodies (b, e, h) and p75NTR monoclonal antibodies (c, f, i). Single optical sections were analyzed by use of a confocal microscope. C, Longitudinal cryosections of distal nerve stumps 1 week (a–c) and 3 weeks (d–f) after nerve crush and from unoperated littermates (g–i) were stained with AN2 polyclonal antibodies (b, e, h) and MAG monoclonal antibodies (c, f, i). Single optical sections were analyzed by use of a confocal microscope. a, b, and d in_B_ and C show the corresponding phase-contrast pictures. Scale bars: B, C, a–c, d–f, g–i, 10 μm. w, Week.
Fig. 7.
The AN2 protein is neither strongly supportive nor repulsive for neurite outgrowth from DRG explants. A, The coating efficiency on PLL-coated cell culture dishes was determined by ELISA. a, The binding efficiency of AN2 developed with AN2 polyclonal antibodies (pcAN2) is shown.b, The binding efficiency of AN2 in the presence of 5 μg/ml LN developed with AN2 polyclonal antibodies (pcAN2) is shown. c, The binding efficiency of LN in the presence of an increasing amount of AN2 developed with LN polyclonal antibodies (anti-LN) is shown. The concentration of the protein solutions that gives a saturating coating on PLL
Fig. 8.
The expression of the AN2 protein in the PMP-22-transgenic rats is increased compared with that in wild-type animals. A, Lysates of sciatic nerves of homozygous (ho), heterozygous (he), and wild-type (wt) PMP-22-transgenic rats (20 μg protein/lane) were separated by gel electrophoresis, blotted, and incubated with AN2 polyclonal antibodies. The same blot was stripped and reprobed with monoclonal antibodies against α-tubulin. B, Longitudinal cryosections of snap-frozen sciatic nerve from homozygous (a–c) and wild-type (d–f) littermates of the same age (4 months) were stained with AN2 polyclonal antibodies (b, e) and MAG monoclonal antibodies (c, f). a and d are the corresponding phase-contrast pictures. Single optical sections were analyzed by use of a confocal microscope. C, Electron microscopic picture of a sciatic nerve from a wild-type littermate of a PMP-22-transgenic rat shows that the nerve contains mainly myelinated axons. D, Electron microscopic picture of a sciatic nerve from a homozygous PMP-22-transgenic rat shows that the nerve mainly contains SCs and axons in a 1:1 relationship and only a few endoneurial fibroblasts, similar to the wild-type littermate (C). Scale bars: B, 10 μm;C, D, 5 μm.
Fig. 9.
Peptide masses obtained by MALDI MS analysis matching with tryptic fragments of the rat NG2 proteoglycan and distribution of the peptide masses along the amino acid sequence of NG2. A, Peptide masses that were obtained by tryptic digestion of the immunoaffinity-purified AN2 protein and subsequent MALDI MS analysis were analyzed with the help of the search program ProFound. Shown are only those peptide masses (m/z submitted) that match with the rat NG2 proteoglycan (MH+ matched). The_column_ headed Delta ppm gives the difference between submitted and matching peptide masses in parts per million (ppm). The amino acid positions of the NG2 peptides are indicated by start and end. The_column_ headed Peptide Sequence is the matching NG2 sequence. Parenthesis indicate the amino acids preceding and following the sequence obtained from the mass determination.Modification indicates that oxidated methionine (Met-ox) residues were also found, yielding two peptide masses matching with one fragment. Peptide sequences obtained by Edman sequencing that matched exactly the published NG2 amino acid sequence are shown bold and underlined.B, The peptide masses of the tryptic AN2 fragments are distributed evenly over the entire ectodomain of NG2 [amino acids (aa) 0–2224]. The bottom of the _x_-axis represents a one-dimensional projection of the data. No peptide fragments matching the transmembrane (aa 2225–2249) or the cytoplasmic (aa 2250–2326) domain were obtained in the mass spectrum.
Fig. 10.
Expression of the AN2 protein in the Schwann cell lineage. The AN2 protein is expressed by immature, promyelinating, and a subset of nonmyelinating SCs. The SC precursor is also AN2 positive. Adapted with modifications from Zorick and Lemke (1996) and Jessen and Mirsky (1999).
Similar articles
- P0 mRNA expression in cultures of Schwann cells and neurons that lack basal lamina and myelin.
Brunden KR, Brown DT. Brunden KR, et al. J Neurosci Res. 1990 Oct;27(2):159-68. doi: 10.1002/jnr.490270206. J Neurosci Res. 1990. PMID: 1701492 - The alpha-chemokine CXCL14 is up-regulated in the sciatic nerve of a mouse model of Charcot-Marie-Tooth disease type 1A and alters myelin gene expression in cultured Schwann cells.
Barbaria EM, Kohl B, Buhren BA, Hasenpusch-Theil K, Kruse F, Küry P, Martini R, Müller HW. Barbaria EM, et al. Neurobiol Dis. 2009 Mar;33(3):448-58. doi: 10.1016/j.nbd.2008.11.014. Epub 2008 Dec 10. Neurobiol Dis. 2009. PMID: 19111616 - SpL201: a conditionally immortalized Schwann cell precursor line that generates myelin.
Lobsiger CS, Smith PM, Buchstaller J, Schweitzer B, Franklin RJ, Suter U, Taylor V. Lobsiger CS, et al. Glia. 2001 Oct;36(1):31-47. doi: 10.1002/glia.1093. Glia. 2001. PMID: 11571782 - Regulation of myelin-specific gene expression. Relevance to CMT1.
Kamholz J, Awatramani R, Menichella D, Jiang H, Xu W, Shy M. Kamholz J, et al. Ann N Y Acad Sci. 1999 Sep 14;883:91-108. Ann N Y Acad Sci. 1999. PMID: 10586235 Review. - AN2, the mouse homologue of NG2, is a surface antigen on glial precursor cells implicated in control of cell migration.
Stegmüller J, Schneider S, Hellwig A, Garwood J, Trotter J. Stegmüller J, et al. J Neurocytol. 2002 Jul-Aug;31(6-7):497-505. doi: 10.1023/a:1025743731306. J Neurocytol. 2002. PMID: 14501219 Review.
Cited by
- Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition.
Jones LL, Sajed D, Tuszynski MH. Jones LL, et al. J Neurosci. 2003 Oct 15;23(28):9276-88. doi: 10.1523/JNEUROSCI.23-28-09276.2003. J Neurosci. 2003. PMID: 14561854 Free PMC article. - Inhibitors of myelination: ECM changes, CSPGs and PTPs.
Harlow DE, Macklin WB. Harlow DE, et al. Exp Neurol. 2014 Jan;251:39-46. doi: 10.1016/j.expneurol.2013.10.017. Epub 2013 Nov 4. Exp Neurol. 2014. PMID: 24200549 Free PMC article. - Cancer immunotherapy targeting the high molecular weight melanoma-associated antigen protein results in a broad antitumor response and reduction of pericytes in the tumor vasculature.
Maciag PC, Seavey MM, Pan ZK, Ferrone S, Paterson Y. Maciag PC, et al. Cancer Res. 2008 Oct 1;68(19):8066-75. doi: 10.1158/0008-5472.CAN-08-0287. Cancer Res. 2008. PMID: 18829565 Free PMC article. - Leptin-receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow.
Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Zhou BO, et al. Cell Stem Cell. 2014 Aug 7;15(2):154-68. doi: 10.1016/j.stem.2014.06.008. Epub 2014 Jun 19. Cell Stem Cell. 2014. PMID: 24953181 Free PMC article. - Intrinsic mutant HTT-mediated defects in oligodendroglia cause myelination deficits and behavioral abnormalities in Huntington disease.
Ferrari Bardile C, Garcia-Miralles M, Caron NS, Rayan NA, Langley SR, Harmston N, Rondelli AM, Teo RTY, Waltl S, Anderson LM, Bae HG, Jung S, Williams A, Prabhakar S, Petretto E, Hayden MR, Pouladi MA. Ferrari Bardile C, et al. Proc Natl Acad Sci U S A. 2019 May 7;116(19):9622-9627. doi: 10.1073/pnas.1818042116. Epub 2019 Apr 23. Proc Natl Acad Sci U S A. 2019. PMID: 31015293 Free PMC article.
References
- Adlkofer K, Lai C. Role of neuregulins in glial cell development. Glia. 2000;29:104–111. - PubMed
- Aguayo A, David S, Richardson P, Bray G. Axon elongation in peripheral and central nervous system transplants. Adv Cell Neurobiol. 1982;3:215–234.
- Amberger VR, Avellanan-Adalid V, Hensel T, Baron-Van Evercooren A, Schwab M. Oligodendrocyte-type 2 astrocyte progenitors use a metalloprotease to spread and migrate on CNS myelin. Eur J Neurosci. 1997;9:151–162. - PubMed
- Anton ES, Sandrock AW, Jr, Matthew WD. Merosin promotes neurite growth and Schwann cell migration in vitro and nerve regeneration in vivo: evidence using an antibody to merosin, ARM-1. Dev Biol. 1994;164:133–146. - PubMed
- Brockes JP, Fields KL, Raff MC. Studies on cultured rat Schwann cells. I. Establishment of purified populations from cultures of peripheral nerve. Brain Res. 1979;165:105–118. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases