The NK1 receptor is essential for the full expression of noxious inhibitory controls in the mouse - PubMed (original) (raw)
The NK1 receptor is essential for the full expression of noxious inhibitory controls in the mouse
H Bester et al. J Neurosci. 2001.
Abstract
Behavioral analysis of the NK1 receptor gene knock-out (NK1-/-) mouse indicated that substance P was closely involved in orchestrating the physiological and behavioral response of the animal to major environmental stressors. In particular, endogenous pain control mechanisms, such as stress-induced analgesia were substantially impaired in mutant mice, suggesting a reduction in descending inhibitory controls to the spinal cord from the brainstem. To directly test the integrity of descending controls in NK1-/- mice, we have analyzed c-Fos expression in laminae I-II of the lumbar and cervical cord and in the rostral ventromedial medulla in an experimental paradigm known to require recruitment of descending inhibitory controls. Anesthetized mice were stimulated with water at 50 degrees C either on their forepaw, hindpaw, or on both the hindpaw plus forepaw concurrently. Wild-type mice, naive or treated with an NK1 antagonist (RP67580) or its inactive isomer (RP68651), were compared with NK1-/- mice. C-Fos expression at the lumbar laminae I-II level was significantly reduced, whereas it was significantly greater in the raphe magnus and pallidus nuclei in the double stimulation situation in wild-type compared with NK1-/- mice. Blocking the NK1 receptor pharmacologically reproduced, in an enantiomere-selective manner, the data from NK1-/- mice, with no evidence for recruitment of descending inhibition at the lumbar cord level after forepaw stimulation. The present study demonstrates that the NK1 receptor is essential for the full development of noxiously evoked descending inhibition.
Figures
Fig. 1.
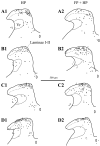
Representative examples of camera lucida drawings of Fos-IR neurons (black dots) in the lumbar dorsal horn ipsilateral to the stimulus in the hindpaw-stimulated animals (HP column) and in the forepaw plus hindpaw-stimulated animals (FP + HP column). A, Wild-type animals. _B,_NK1−/− mice. C, Wild-type animals injected with the RP67580 NK1 antagonist. D, Wild-type mice injected with the NK1 antagonist isomer RP68651. Vr, Reticular part of the lamina V. Laminae I-II, Laminae I and II of the dorsal horn. Scale bar, 500 μm.
Fig. 2.
Photomicrographs of the lumbar dorsal horn (segments L4/5) ipsilateral to the stimulation. Note the reduction in the number of Fos-IR reactive neurons in wild-type animals (A, B) between a hindpaw stimulation only (A) and a forepaw plus hindpaw stimulation (B), and the absence of such an effect in NK1−/− (C, D). Scale bar, 400 μm.
Fig. 3.
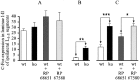
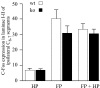
Histograms of the average number per section of Fos-IR neurons evoked in the lumbar laminae I-II, in the HP (A), FP (B), and FP + HP (C) situations. ★p < 0.05; ★★p < 0.01; ★★★p < 0.001.
Fig. 4.
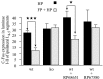
Histograms of the average number per section of Fos-IR neurons evoked in the lumbar laminae I-II, comparing the HP and the FP + HP situations. ★p < 0.05; ★★★p < 0.001.
Fig. 5.
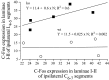
Relationship between lumbar and cervical c-Fos expression in the FP + HP situation. Regression plots and correlation lines and functions of the lumbar against cervical numbers of Fos-IR neurons in laminae I-II.
Fig. 6.
Histograms of the average number per section of Fos-IR neurons evoked in the cervical laminae I-II, in the HP, FP, and FP + HP situations.
Fig. 7.
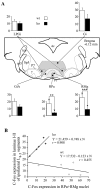
C-Fos expression in the rostral ventromedial medulla and its influence on the c-Fos expression in lumbar laminae I-II in the FP + HP situation. A, Histograms of average numbers of Fos-IR neurons observed in different raphe nuclei viewed on a schematic representation of the medulla from the Franklin and Watson (1997) atlas. ★★p < 0.01; ★★★p < 0.001. _Gi,_Gigantocellular reticular; GiA, gigantocellular reticular pars α; LPGi, lateral paragigantocellular reticular; RMg, raphe magnus; RPa, raphe pallidus. B, Correlation plots of the numbers of Fos-IR neurons in the RPa and RMg raphe nuclei. _Filled symbols_correspond to NK1−/− mice; open symbols correspond to wild-type mice.
Similar articles
- Physiological contributions of neurokinin 1 receptor activation, and interactions with NMDA receptors, to inflammatory-evoked spinal c-Fos expression.
Chapman V, Buritova J, Honoré P, Besson JM. Chapman V, et al. J Neurophysiol. 1996 Sep;76(3):1817-27. doi: 10.1152/jn.1996.76.3.1817. J Neurophysiol. 1996. PMID: 8890294 - Superficial NK1-expressing neurons control spinal excitability through activation of descending pathways.
Suzuki R, Morcuende S, Webber M, Hunt SP, Dickenson AH. Suzuki R, et al. Nat Neurosci. 2002 Dec;5(12):1319-26. doi: 10.1038/nn966. Nat Neurosci. 2002. PMID: 12402039 - C-FOS expression in the rat brain in response to substance P and neurokinin B.
Spitznagel H, Baulmann J, Blume A, Unger T, Culman J. Spitznagel H, et al. Brain Res. 2001 Oct 19;916(1-2):11-21. doi: 10.1016/s0006-8993(01)02858-x. Brain Res. 2001. PMID: 11597586 - NK1 (substance P) receptor antagonists--why are they not analgesic in humans?
Hill R. Hill R. Trends Pharmacol Sci. 2000 Jul;21(7):244-6. doi: 10.1016/s0165-6147(00)01502-9. Trends Pharmacol Sci. 2000. PMID: 10871891 Review. - [Why are substance P(NK1)-receptor antagonists ineffective in pain treatment?].
Herbert MK, Holzer P. Herbert MK, et al. Anaesthesist. 2002 Apr;51(4):308-19. doi: 10.1007/s00101-002-0296-7. Anaesthesist. 2002. PMID: 12063723 Review. German.
Cited by
- Non-peptidergic small diameter primary afferents expressing VGluT2 project to lamina I of mouse spinal dorsal horn.
Clarke JN, Anderson RL, Haberberger RV, Gibbins IL. Clarke JN, et al. Mol Pain. 2011 Dec 8;7:95. doi: 10.1186/1744-8069-7-95. Mol Pain. 2011. PMID: 22152428 Free PMC article. - Neurokinin-1 receptors (NK1R:s), alcohol consumption, and alcohol reward in mice.
Thorsell A, Schank JR, Singley E, Hunt SP, Heilig M. Thorsell A, et al. Psychopharmacology (Berl). 2010 Mar;209(1):103-11. doi: 10.1007/s00213-010-1775-1. Epub 2010 Jan 30. Psychopharmacology (Berl). 2010. PMID: 20112009 - Neurokinin-1 projection cells in the rat dorsal horn receive synaptic contacts from axons that possess alpha2C-adrenergic receptors.
Olave MJ, Maxwell DJ. Olave MJ, et al. J Neurosci. 2003 Jul 30;23(17):6837-46. doi: 10.1523/JNEUROSCI.23-17-06837.2003. J Neurosci. 2003. PMID: 12890778 Free PMC article. - Tachykinins modulate nociceptive responsiveness and sensitization: In vivo electrical characterization of primary sensory neurons in tachykinin knockout (Tac1 KO) mice.
Gutierrez S, Alvarado-Vázquez PA, Eisenach JC, Romero-Sandoval EA, Boada MD. Gutierrez S, et al. Mol Pain. 2019 Jan-Dec;15:1744806919845750. doi: 10.1177/1744806919845750. Mol Pain. 2019. PMID: 31012376 Free PMC article. - Preprotachykinin A is expressed by a distinct population of excitatory neurons in the mouse superficial spinal dorsal horn including cells that respond to noxious and pruritic stimuli.
Gutierrez-Mecinas M, Bell AM, Marin A, Taylor R, Boyle KA, Furuta T, Watanabe M, Polgár E, Todd AJ. Gutierrez-Mecinas M, et al. Pain. 2017 Mar;158(3):440-456. doi: 10.1097/j.pain.0000000000000778. Pain. 2017. PMID: 27902570 Free PMC article.
References
- Abbadie C, Honoré P, Fournie-Zaluski MC, Roques BP, Besson JM. Effects of opioids and non-opioids on c-Fos-like immunoreactivity induced in rat lumbar spinal cord neurons by noxious heat stimulation. Eur J Pharmacol. 1994;258:215–227. - PubMed
- Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–209. - PubMed
- Battaglia G, Rustioni A. Coexistence of glutamate and substance P in dorsal root ganglion neurons of the rat and monkey. J Comp Neurol. 1988;277:302–312. - PubMed
- Beitz AJ. The nuclei of origin of brain stem enkephalin and substance P projections to the rodent nucleus raphe magnus. Neuroscience. 1982;7:2753–2768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases