XMAP215 regulates microtubule dynamics through two distinct domains - PubMed (original) (raw)
XMAP215 regulates microtubule dynamics through two distinct domains
A V Popov et al. EMBO J. 2001.
Abstract
XMAP215 belongs to a family of proteins involved in the regulation of microtubule dynamics. In this study we analyze the function of different parts of XMAP215 in vivo and in Xenopus egg extracts. XMAP215 has been divided into three fragments, FrN, FrM and FrC (for N-terminal, middle and C-terminal, respectively). FrN co-localizes with microtubules in egg extracts but not in cells, FrC co- localizes with microtubules and centrosomes both in egg extracts and in cells, while FrM does not co- localize with either centrosomes or microtubules. In Xenopus egg extracts, FrN stimulates microtubule growth at plus-ends by inhibiting catastrophes, while FrM has no effect, and FrC suppresses microtubule growth by promoting catastrophes. Our results suggest that XMAP215 is targeted to centrosomes and microtubules mainly through its C-terminal domain, while the evolutionarily conserved N-terminal domain contains its microtubule-stabilizing activity.
Figures
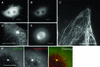
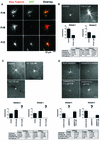
Fig. 1. Immunoelectron microscopic localization of XMAP215. (A) Electron microscopy on XL177 cells. In cultured epithelial cells, anti-N-terminal XMAP215 antibodies stain microtubules and, to a lesser extent, pericentriolar material. (B) In asters, assembled in vitro using mitotic Xenopus egg extracts and human KE37 cell line centrosomes, XMAP215 is localized on microtubules and pericentriolar material. Arrows indicate gold particles associated with pericentriolar material. Scale bar, 200 nm.
Fig. 2. GFP–XMAP215 associates with microtubules and centrosomes in interphase and mitosis. (A) A live XL177 cell electroporated with GFP–XMAP215. Note that cultured Xenopus cells frequently have more than one nucleus. (B) Same cell as in (A), showing the plane distal from the glass coverslip. The bright fluorescent spot is the centrosome. (C) High-magnification image of a live cell, showing GFP–XMAP215 associated with interphase microtubules at the periphery of the cell. (D) High-magnification image showing GFP–XMAP215 on the centrosome and proximal to centrosome microtubules. (E) In mitosis, GFP–XMAP215 stains the mitotic spindle and spindle poles. (F) Immunolocalization of XMAP215 in fixed XL177 cells after nocodazole-induced depolymerization of microtubules. Scale bars, 10 µm.
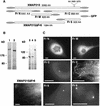
Fig. 3. Intracellular localization of the XMAP215 fragments expressed as GFP fusions. (A) Schematic representation of fragments and GFP fusion constructs of XMAP215 and five XMAP215 fragments. Full-length XMAP215, FrN and FrC were separately expressed as either N- or C-terminal fusions with GFP to exclude artifacts created by hindrance of the GFP tag. Sizes of fragments in amino acids (AA) are shown excluding GFP. (B) Immunoblotting analysis showing protein bands corresponding to the full-length GFP–XMAP215 (1), GFP–XMAP215_ΔFr6 (2), FrN–GFP (3), FrM–GFP (4) and FrC–GFP (5). Lysates from transfected cells were resolved on a polyacrylamide gel, blotted and probed with anti-GFP antibodies. Molecular weight markers are shown on the left-hand side. (C) Expression of the GFP fusions of FrN, FrM, FrC, XMAP215_ΔFr6 and Fr6 in live XL177 cells. Scale bars, 10 µm.
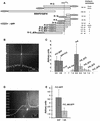
Fig. 4. The centrosome-binding site is localized at the C-terminal part of XMAP215. (A) Schematic representation of the GFP fusion constructs of XMAP215 designed to map the centrosome-binding domain. FrC, Fr9 and Fr10 were separately expressed as either N- or C-terminal fusions with GFP to exclude artifacts created by hindrance of the GFP tag. KKIGS1874K is a six amino acid sequence which was mutated in FrC–GFP to assess its role in centrosome binding. A vertical line through the C-terminus of XMAP215 shows the position of the KKIGSK sequence in different constructs. Relative intensity of centrosome staining indicated on the right is based on the results shown in (C). (B) Intensity profile of GFP fluorescence in a cell expressing FrC–GFP. (C) Ratios of intensities of centrosome/cytoplasm in live XL177 cells expressing different C-terminal fragments (see A). To calculate the ratios, the camera background was first subtracted from cytoplasm and centrosome fluorescence. The scale of the _y_-axis begins at 1, representing a cell in which no difference in fluorescence intensity between centrosome and cytoplasm exists, for example, in cells transfected with GFP fusions of Fr9, Fr10 and FrC_ΔC6. For each bar, 20 cells were assessed in a blind assay. Values of intensity ratios are indicated under the columns. GFP–FrC and FrC–GFP represent fusion proteins with N- or C-terminal positions of GFP. (D) Intensity profile of GFP fluorescence in a live XL177 cell expressing FrC_M6–GFP, where M6 stands for the mutation introduced into FrC with amino acids 1870–1875 (KKIGSK) replaced by six irrelevant amino acids (NGYLAQ). Note that the level of GFP fluorescence in the cytoplasm is higher than in (B). (E) Ratios of intensities of centrosome/cytoplasm in live XL177 cells, electroporated with FrC–GFP and FrC_M6–GFP. For each bar, 40 cells were measured. Note that the centrosome/cytoplasm fluorescence ratio of FrC–GFP in this experiment is very close to the data in (C). The scale of the _y_-axis begins at 1 (as in C).
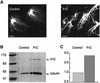
Fig. 5. The C-terminal fragment of XMAP215 has the highest affinity for pure tubulin microtubules. (A) GFP fusion proteins of FrN, FrM and FrC were incubated with pure tubulin microtubules and sedimented without fixation. CY5-labeled microtubule seeds are shown in a blue pseudocolor. Note that all three of the fragments studied are associated with microtubules whilst GFP alone does not bind to microtubules. (B) Microtubule spin-down assay. c-_myc_-tagged FrN, FrM and FrC were incubated with 1 µM pure tubulin microtubules. After sedimentation, pellets and supernatants were analyzed by SDS–electrophoresis followed by Coomassie Blue staining. BSA was present in reactions to prevent precipitation and/or absorption of XMAP215 fragments on the walls of test tubes. Note that the FrN band (62 kDa) is just under the BSA band. (C) Immunoblotting results of the same experiment shown in (B).
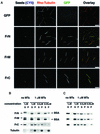
Fig. 6. N- and C-terminal parts of XMAP215 bind to centrosomal asters in egg extracts and have opposite effects on microtubule dynamics. (A) Only GFP–FrN and GFP–FrC bind to the microtubule asters assembled in a mitotic Xenopus egg extract. Asters were assembled in the presence of rhodamine–tubulin and 0.1 µM recombinant XMAP215 fragments fused to GFP. Scale bar, 10 µm. (B) Addition of FrN to mitotic egg extract reduces the number of catastrophes. At the top are the images of microtubule asters as observed in mitotic Xenopus egg extracts during recording by fluorescence video microscopy. Results in the table summarize two separate experiments performed in two different extracts. Control reactions show microtubule dynamics after the addition of the buffer. The final concentration of FrN in the reaction was 2.7 µM. (C) FrC augments the catastrophe rate in mitotic egg extracts. Note that at 1.8 µM FrC practically no microtubules are visible (corresponding catastrophe rate is shown in the chart as ?). Therefore, effects of FrC on microtubule dynamics were recorded at a final concentration of 0.6 µM. Results from two different extracts are presented. (D) The phenotype of the XMAP215 depletion can be reversed by addition of FrN. Results from two different extracts are presented. Note that at 1 µM, FrN partially restores microtubule dynamics to the level of the mock-depleted extract, but at 2.7 µM the number of catastrophes was even lower than before the depletion of XMAP215. Scale bar, 5 µm. Note that the number of rescues is not indicated in the tables of (B), (C) and (D) because in most cases the number of events observed was too low.
Fig. 7. FrC does not have an intrinsic microtubule-depolymerizing activity. (A) FrC was added to preformed microtubule asters growing from centrosomes in the presence of pure tubulin. Note the extensive bundling of microtubules in the presence of FrC. (B) Coomassie-stained gel with samples from the experiment shown in (A). (C) Quantification of the tubulin protein bands shown in (B). The mass of polymerized tubulin in the asters increased 2.8-fold in the presence of 2 µM FrC compared with the control reaction.
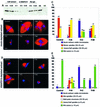
Fig. 8. The N-terminal part of XMAP215 partially rescues mitotic spindle formation in XMAP215-depleted extracts. The C-terminal part of XMAP215 inhibits microtubule growth around sperm nuclei. (A) Immunoblot with anti-XMAP215 antibodies showing the efficiency of XMAP215 depletion from Xenopus egg mitotic extracts. Lanes 1–3 represent 0.5, 0.25 and 0.1 µl of the extract resolved on a 6% SDS–gel. Lanes 4–5 show 0.5 and 0.25 µl of an XMAP215-depleted extract (ΔXMAP215). Lanes 6–7 show 0.5 and 0.25 µl of a mock-depleted extract (Rb IgG, non-immune rabbit serum IgG). XE, eluate from Dynabeads loaded with anti-XMAP215 antibodies after depletion. ME, eluate from Dynabeads loaded with Rb IgG after mock depletion. (B) Formation of mitotic spindles in the cycled extracts after the addition of recombinant XMAP215 fragments. Most frequent phenotypes are shown. (C) Quantification of the phenotypes shown in (B). More than one hundred nuclei per reaction were observed and the microtubule structures associated with these nuclei were classified. (D) Formation of spindles and other structures in the cycled XMAP215-depleted extracts in the presence of recombinant XMAP215 fragments. (E) Quantification of the phenotypes shown in (D). More than one hundred nuclei per reaction were estimated. Scale bar, 10 µm.
Fig. 9. Domain organization of some members of the XMAP215 family of proteins. On the top is a dot plot of the XMAP215 protein sequence compared with Zyg-9. Two regions of homology were detected: the N-terminal domain (1) and a more distal domain (2). The N-terminal domain is duplicated in Zyg-9. This plot was used as a basis for splitting XMAP215 into three major fragments: FrN, FrM and FrC. Below are some other members of the XMAP215 family with corresponding homology domains. p93dis1 (as well as STU2 protein from S.cerevisiae) is considerably shorter than XMAP215 and contains only domain 1, which is 31% identical to domain 1 in XMAP215. Human ch-TOG (as well as Msps protein from Drosophila and DdCP224 from D.discoideum) contains both domain 1 and 2 and is homologous to XMAP215 over the whole length of the protein. ch-TOG has 76% overall identity with XMAP215. Finally, Zyg-9 is shorter than ch-TOG, msps and DdCP224, and has two copies of domain 1 and one copy of domain 2. The first and the second domain 1 in Zyg-9 show 41 and 45% identity to the corresponding domain 1 in XMAP215.
Fig. 10. Model explaining the activity of the XMAP215 fragments in egg extracts. XMAP215 binds to microtubules and prevents the induction of microtubule catastrophes by Kin I kinesins. The N-terminal fragment of XMAP215 (FrN) opposes the Kin I kinesin activity. The C-terminal fragment of XMAP215 (FrC) displaces the endogenous XMAP215, and thus exposes the plus-ends of microtubules to the destabilizing activity of the catastrophic kinesins.
Similar articles
- XMAP215 is required for the microtubule-nucleating activity of centrosomes.
Popov AV, Severin F, Karsenti E. Popov AV, et al. Curr Biol. 2002 Aug 6;12(15):1326-30. doi: 10.1016/s0960-9822(02)01033-3. Curr Biol. 2002. PMID: 12176362 - Control of microtubule dynamics by the antagonistic activities of XMAP215 and XKCM1 in Xenopus egg extracts.
Tournebize R, Popov A, Kinoshita K, Ashford AJ, Rybina S, Pozniakovsky A, Mayer TU, Walczak CE, Karsenti E, Hyman AA. Tournebize R, et al. Nat Cell Biol. 2000 Jan;2(1):13-9. doi: 10.1038/71330. Nat Cell Biol. 2000. PMID: 10620801 - Xenopus NEDD1 is required for microtubule organization in Xenopus egg extracts.
Liu L, Wiese C. Liu L, et al. J Cell Sci. 2008 Mar 1;121(Pt 5):578-89. doi: 10.1242/jcs.018937. Epub 2008 Feb 5. J Cell Sci. 2008. PMID: 18252801 - The role of TOG domains in microtubule plus end dynamics.
Slep KC. Slep KC. Biochem Soc Trans. 2009 Oct;37(Pt 5):1002-6. doi: 10.1042/BST0371002. Biochem Soc Trans. 2009. PMID: 19754440 Review. - XMAP215: a key component of the dynamic microtubule cytoskeleton.
Kinoshita K, Habermann B, Hyman AA. Kinoshita K, et al. Trends Cell Biol. 2002 Jun;12(6):267-73. doi: 10.1016/s0962-8924(02)02295-x. Trends Cell Biol. 2002. PMID: 12074886 Review.
Cited by
- The XMAP215 family drives microtubule polymerization using a structurally diverse TOG array.
Fox JC, Howard AE, Currie JD, Rogers SL, Slep KC. Fox JC, et al. Mol Biol Cell. 2014 Aug 15;25(16):2375-92. doi: 10.1091/mbc.E13-08-0501. Epub 2014 Jun 25. Mol Biol Cell. 2014. PMID: 24966168 Free PMC article. - The transcriptional repressor Kaiso localizes at the mitotic spindle and is a constituent of the pericentriolar material.
Soubry A, Staes K, Parthoens E, Noppen S, Stove C, Bogaert P, van Hengel J, van Roy F. Soubry A, et al. PLoS One. 2010 Feb 15;5(2):e9203. doi: 10.1371/journal.pone.0009203. PLoS One. 2010. PMID: 20169156 Free PMC article. - TOGp, the human homolog of XMAP215/Dis1, is required for centrosome integrity, spindle pole organization, and bipolar spindle assembly.
Cassimeris L, Morabito J. Cassimeris L, et al. Mol Biol Cell. 2004 Apr;15(4):1580-90. doi: 10.1091/mbc.e03-07-0544. Epub 2004 Jan 12. Mol Biol Cell. 2004. PMID: 14718566 Free PMC article. - The viral protein gp120 decreases the acetylation of neuronal tubulin: potential mechanism of neurotoxicity.
Avdoshina V, Caragher SP, Wenzel ED, Taraballi F, Mocchetti I, Harry GJ. Avdoshina V, et al. J Neurochem. 2017 May;141(4):606-613. doi: 10.1111/jnc.14015. Epub 2017 Apr 6. J Neurochem. 2017. PMID: 28295345 Free PMC article. - Fission yeast ch-TOG/XMAP215 homologue Alp14 connects mitotic spindles with the kinetochore and is a component of the Mad2-dependent spindle checkpoint.
Garcia MA, Vardy L, Koonrugsa N, Toda T. Garcia MA, et al. EMBO J. 2001 Jul 2;20(13):3389-401. doi: 10.1093/emboj/20.13.3389. EMBO J. 2001. PMID: 11432827 Free PMC article.
References
- Andersen S.S., Buendia,B., Dominguez,J.E., Sawyer,A. and Karsenti,E. (1994) Effect on microtubule dynamics of XMAP230, a microtubule-associated protein present in Xenopus laevis eggs and dividing cells [published erratum appears in J. Cell Biol., 1995, 128, following 988]. J. Cell Biol., 127, 1289–1299. - PMC - PubMed
- Belmont L. and Mitchison,T.J. (1996) Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell, 84, 623–631. - PubMed
- Chang P. and Stearns,T. (2000) δ-tubulin and ε-tubulin: two new human centrosomal tubulins reveal new aspects of centrosome structure and function. Nature Cell Biol., 2, 30–35. - PubMed
- Chapin S.J. and Bulinski,J.C. (1992) Microtubule stabilization by assembly-promoting microtuble-associated proteins: a repeat per formance. Cell Motil. Cytoskeleton, 23, 236–243. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources