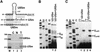A small nucleolar guide RNA functions both in 2'-O-ribose methylation and pseudouridylation of the U5 spliceosomal RNA - PubMed (original) (raw)
A small nucleolar guide RNA functions both in 2'-O-ribose methylation and pseudouridylation of the U5 spliceosomal RNA
B E Jády et al. EMBO J. 2001.
Abstract
In eukaryotes, two distinct classes of small nucleolar RNAs (snoRNAs), namely the fibrillarin-associated box C/D snoRNAs and the Gar1p-associated box H/ACA snoRNAs, direct the site-specific 2'-O-ribose methylation and pseudouridylation of ribosomal RNAs (rRNAs), respectively. We have identified a novel evolutionarily conserved snoRNA, called U85, which possesses the box elements of both classes of snoRNAs and associates with both fibrillarin and Gar1p. In vitro and in vivo pseudouridylation and 2'-O-methylation experiments provide evidence that the U85 snoRNA directs 2'-O-methylation of the C45 and pseudouridylation of the U46 residues in the invariant loop 1 of the human U5 spliceosomal RNA. The U85 is the first example of a snoRNA that directs modification of an RNA polymerase II-transcribed spliceosomal RNA and that functions both in RNA pseudouridylation and 2'-O-methylation.
Figures
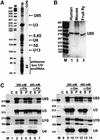
Fig. 1. Characterization of U85 snoRNA. (A) Human fibrillarin-associated snoRNAs. RNAs immunopreciptated with monoclonal anti-fibrillarin antibody (72B9) were 3′ end-labelled and fractionated on a 6% sequencing gel (lane FIB). Lane CON, control precipitation with monoclonal anti-Sp1 antibody. Lane M, size markers. (B) Northern analysis. RNAs from human, mouse and fruit fly cells were fractionated on a 6% denaturing gel and probed with an internally labelled antisense human U85 RNA. (C) Anti-fibrillarin and anti-hGAR1 antibodies recognize the human U85 snoRNP. Extracts prepared from HeLa cells in the presence of 250 or 400 mM NaCl were reacted with anti-fibrillarin (α-Fib) or anti-hGAR1 (α-Gar) antibodies. Distribution of the U85, U3 and U19 snoRNA and the U4 snRNA in the extracts (lanes E) and supernatants (lanes S) or pellets (lanes P) of the immunoprecipitation reactions were determined by RNase A/T1 protections. Lanes C represent control mappings with Escherichia coli tRNA. Lanes M, size markers.
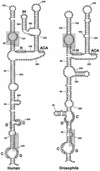
Fig. 2. Proposed secondary structures of the human and fruit fly U85 snoRNAs. The box C, C′, H, ACA, D and D′ motifs are boxed. Other sequences conserved between the two snoRNAs are shaded.
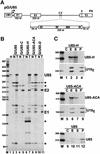
Fig. 3. Processing of the human U85 intronic snoRNA. (A) Structure of the expression constructs used for transfection of COS7 cells. The exons (E1, E2 and E3) and the polyadenylation site (PA) of the human β-globin gene and the promoter region of the cytomegalovirus (CMV) are indicated. Relevant restriction sites are shown (H, _Hin_dIII; C, _Cla_I; X, _Xho_I; E, _Eco_RI). The U85 snoRNA gene inserted into the second intron of the β-globin gene is indicated by open arrow. In the pG/U85-C, pG/U85-H, pG/U85-ACA and pG/U85-D expression constructs, the C, H, ACA or D boxes of U85 were replaced with C residues. (B) RNase A/T1 protection. Simian COS7 cells were transfected with the pG/U85, pG/U85-C, pG/U85-H, pG/U85-ACA or pG/U85-D expression construct. RNAs extracted from transfected (lanes T) or non-transfected (lanes N) cells were mapped with appropriate antisense RNA probes as indicated above the lanes. Lane H, control mapping with HeLa RNA. RNAs protected by the human U85 snoRNA, the first (E1) and second (E2) exon of the globin mRNA are indicated. (C) The U85-H and U85-ACA snoRNAs are not associated with Gar1p. Extracts of COS7 cells transfected with the pG/U85-H, pG/U85-ACA or the pG/U85 expression construct were subjected to immunoprecipitation with anti-hGAR1 antibodies. RNAs recovered from the extracts (E), the supernatant (S) or the pellet (P) of the immunoprecipitation reactions were mapped by RNase A/T1 protection by using RNA probes specific for the U85-H, U85-ACA, U85 and U19 snoRNAs. Lanes C, control mappings with E.coli tRNA. Lanes M, size markers.
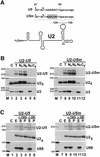
Fig. 4. The U85 snoRNA can direct modification of the U5 snRNA. (A) Potential hydrogen bonding between U85 and U5 RNAs in human and Drosophila cells. The 5′ hairpins in the H/ACA domains of the U85 snoRNAs are schematically represented. Pseudouridines (Ψ) and 2′-_O_-methyl groups (closed circles) in the U5 snRNA are indicated. Nucleotides potentially selected by the U85 snoRNAs are shaded. (B) In vitro pseudouridylation of U5 RNA. In the presence or absence of in vitro synthesized wild-type (U85) or mutant (U85m) human U85 snoRNA, [α-32P]UTP-labelled U5 snRNA was incubated in a HeLa extract (S100+NU) that had been either not treated or treated with micrococcal nuclease (MN). Three RNase T1 fragments of the U5 substrate RNAs were digested with nuclease P1 and analysed by one-dimensional TLC. PhosphorImager quantification revealed that the pseudouridine content of the 13 nucleotide fragment was ∼35% of the theoretical yield. (C) In vitro transcribed U85 and [32P]ATP-labelled U5 RNA were incubated with an MN-treated extract. The 6- (lane 1) and 5-nt (lane 2) RNase CL-3 subfragments of the 13-nt RNase T1 fragment of substrate U5 RNA were digested with RNase T2 and analysed by TLC.
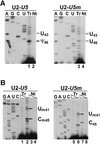
Fig. 5. Expression of chimeric U2 snRNAs in COS7 cells. (A) Schematic structure of the U2–U5 and U2–U5m snRNAs. Sequences of the U5 and U5m tags and positions of pseudouridines (Ψ) and 2′-O_-methylated nucleotides (closed circles) are shown. The authentic U5 sequences are in upper case. Altered nucleotides in the U5m tag are shaded. (B) Subcellular localization of U2–_U5 and U2–U5m RNAs. RNAs extracted either from whole cells (T) or from the nuclear (Nu), nucleoplasmic (Np), nucleolar (No) and cytoplasmic (Cy) fractions of COS7 cells transfected with the pGL/U2-U5 or pGL/U2-U5m construct were mapped by RNase A/T1 protection using antisense RNA probes specific for the U2–U5 and U2–U5m snRNAs, and the U3 snoRNA. Lanes C, control mappings with RNAs from non-transfected cells. Lanes M, size markers. (C) Immunoprecipitation. Extracts obtained from COS7 cells transfected with the pGL/U2-U5 or pGL/U2-U5m construct were precipitated with anti-trimethylguanosine (α-TMG) and anti-SM (α-SM) antibodies. RNAs extracted from cell extracts (E) or from the supernatant (S) and pellet (P) of the immunoprecipitation reactions were mapped by RNase protection by using RNA probes specific for the U2–U5 and U2–U5m snRNAs, and the U69 snoRNA. Lanes C, control mappings with E.coli tRNA.
Fig. 6. Primer extension mapping of pseudouridines and 2′-O_-methylated nucleotides in the U2–_U5 and U2–U5m snRNAs. (A) Mapping of pseudouridines. CMC-alkali-treated cellular RNAs extracted from COS7 cells transfected (Tr) or non-transfected (Nt) with the pGL/U2-U5 and pGL/U2-U5m constructs were analysed by primer extension by using a terminally labelled oligonucleotide primer specific for the U2–U5 and U2–U5m snRNAs. Closed and open arrows indicate the actual and expected positions of pseudouridines, respectively. Lanes A, G, C and U are dideoxy sequencing reactions. (B) Mapping of 2′-_O_-methyl groups. Cellular RNAs isolated from transfected (Tr) or non-transfected (Nt) cells were analysed by primer extension in the presence of 1.0 or 0.004 mM dNTPs as indicated above the lanes.
Fig. 7. Restoration of pseudouridylation and 2′-O_-methylation of the U2–_U5m snRNA. (A) Expression and predicted interaction of the U85m pseudouridylation and 2′-O_-methylation guide snoRNA and the U2–_U5m snRNA. RNAs from COS7 cells transfected (T) and non-transfected (N) with the pG/U85m/U2-U5m expression construct were mapped by RNase protection. Lane M, size markers. (B) Mapping of pseudouridines. CMC-alkali-treated cellular RNAs extracted from COS7 cells expressing the U2–U5m snRNA alone (lane 1) or together with the U85m snoRNA (lane 2) were analysed by primer extension. (C) Mapping of 2′-O_-methylated nucleotides. Distribution of ribose-methylated nucleotides in the U2–_U5m snRNA expressed in the absence (lanes 1 and 2) or presence of the U85m snoRNA (lane 3 and 4) was determined by primer extension analysis.
Similar articles
- A novel snoRNA can direct site-specific 2'-O-ribose methylation of snRNAs in Oryza sativa.
Li W, Jiang G, Huang B, Jin Y. Li W, et al. IUBMB Life. 2005 Mar;57(3):173-9. doi: 10.1080/15216540500090819. IUBMB Life. 2005. PMID: 16036579 - Characterisation of the U83 and U84 small nucleolar RNAs: two novel 2'-O-ribose methylation guide RNAs that lack complementarities to ribosomal RNAs.
Jády BE, Kiss T. Jády BE, et al. Nucleic Acids Res. 2000 Mar 15;28(6):1348-54. doi: 10.1093/nar/28.6.1348. Nucleic Acids Res. 2000. PMID: 10684929 Free PMC article. - A Cajal body-specific pseudouridylation guide RNA is composed of two box H/ACA snoRNA-like domains.
Kiss AM, Jády BE, Darzacq X, Verheggen C, Bertrand E, Kiss T. Kiss AM, et al. Nucleic Acids Res. 2002 Nov 1;30(21):4643-9. doi: 10.1093/nar/gkf592. Nucleic Acids Res. 2002. PMID: 12409454 Free PMC article. - RNA modification in Cajal bodies.
Meier UT. Meier UT. RNA Biol. 2017 Jun 3;14(6):693-700. doi: 10.1080/15476286.2016.1249091. Epub 2016 Oct 24. RNA Biol. 2017. PMID: 27775477 Free PMC article. Review. - Biogenesis and intranuclear trafficking of human box C/D and H/ACA RNPs.
Kiss T, Fayet E, Jády BE, Richard P, Weber M. Kiss T, et al. Cold Spring Harb Symp Quant Biol. 2006;71:407-17. doi: 10.1101/sqb.2006.71.025. Cold Spring Harb Symp Quant Biol. 2006. PMID: 17381323 Review.
Cited by
- Genome-wide determination of RNA stability reveals hundreds of short-lived noncoding transcripts in mammals.
Tani H, Mizutani R, Salam KA, Tano K, Ijiri K, Wakamatsu A, Isogai T, Suzuki Y, Akimitsu N. Tani H, et al. Genome Res. 2012 May;22(5):947-56. doi: 10.1101/gr.130559.111. Epub 2012 Feb 27. Genome Res. 2012. PMID: 22369889 Free PMC article. - Small nucleolar RNA-guided post-transcriptional modification of cellular RNAs.
Kiss T. Kiss T. EMBO J. 2001 Jul 16;20(14):3617-22. doi: 10.1093/emboj/20.14.3617. EMBO J. 2001. PMID: 11447102 Free PMC article. Review. No abstract available. - Identification of 86 candidates for small non-messenger RNAs from the archaeon Archaeoglobus fulgidus.
Tang TH, Bachellerie JP, Rozhdestvensky T, Bortolin ML, Huber H, Drungowski M, Elge T, Brosius J, Hüttenhofer A. Tang TH, et al. Proc Natl Acad Sci U S A. 2002 May 28;99(11):7536-41. doi: 10.1073/pnas.112047299. Proc Natl Acad Sci U S A. 2002. PMID: 12032318 Free PMC article. - RNomics in Drosophila melanogaster: identification of 66 candidates for novel non-messenger RNAs.
Yuan G, Klämbt C, Bachellerie JP, Brosius J, Hüttenhofer A. Yuan G, et al. Nucleic Acids Res. 2003 May 15;31(10):2495-507. doi: 10.1093/nar/gkg361. Nucleic Acids Res. 2003. PMID: 12736298 Free PMC article. - Activation of PARP-1 by snoRNAs Controls Ribosome Biogenesis and Cell Growth via the RNA Helicase DDX21.
Kim DS, Camacho CV, Nagari A, Malladi VS, Challa S, Kraus WL. Kim DS, et al. Mol Cell. 2019 Sep 19;75(6):1270-1285.e14. doi: 10.1016/j.molcel.2019.06.020. Epub 2019 Jul 24. Mol Cell. 2019. PMID: 31351877 Free PMC article.
References
- Bakin A. and Ofengand,J. (1993) Four newly located pseudouridylate residues in Escherichia coli 23S ribosomal RNA are all at the peptidyltransferase center: analysis by the application of a new sequencing technique. Biochemistry, 32, 9754–9762. - PubMed
- Balakin AG., Smith,L. and Fournier,M.J. (1996) The RNA world of the nucleolus: two major families of small RNAs defined by different box elements with related functions. Cell, 86, 823–834. - PubMed
- Cavaillé J., Nicoloso,M. and Bachellerie,J.-P. (1996) Targeted ribose methylation of RNA in vivo directed by tailored antisense RNA guides. Nature, 383, 732–735. - PubMed
- Ganot P., Bortolin,M.-L. and Kiss,T. (1997a) Site-specific pseudouridine formation in preribosomal RNA is guided by small nucleolar RNAs. Cell, 89, 799–809. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases