Disulfide bond formation in secreton component PulK provides a possible explanation for the role of DsbA in pullulanase secretion - PubMed (original) (raw)
Disulfide bond formation in secreton component PulK provides a possible explanation for the role of DsbA in pullulanase secretion
A P Pugsley et al. J Bacteriol. 2001 Feb.
Abstract
When expressed in Escherichia coli, the 15 Klebsiella oxytoca pul genes that encode the so-called Pul secreton or type II secretion machinery promote pullulanase secretion and the assembly of one of the secreton components, PulG, into pili. Besides these pul genes, efficient pullulanase secretion also requires the host dsbA gene, encoding a periplasmic disulfide oxidoreductase, independently of disulfide bond formation in pullulanase itself. Two secreton components, the secretin pilot protein PulS and the minor pseudopilin PulK, were each shown to posses an intramolecular disulfide bond whose formation was catalyzed by DsbA. PulS was apparently destabilized by the absence of its disulfide bond, whereas PulK stability was not dramatically affected either by a dsbA mutation or by the removal of one of its cysteines. The pullulanase secretion defect in a dsbA mutant was rectified by overproduction of PulK, indicating reduced disulfide bond formation in PulK as the major cause of the secretion defect under the conditions tested (in which PulS is probably present in considerable excess of requirements). PulG pilus formation was independent of DsbA, probably because PulK is not needed for piliation.
Figures
FIG. 1
Localization of PulS, PulK, and PulG proteins by flotation sucrose gradient centrifugation of membranes from E. coli K-12 PAP7460(pCHAP231) grown in medium containing 0.4% maltose. Fractions collected from the gradients were examined by SDS-PAGE (11.3% acrylamide, 8 M urea) and immunoblotting with the appropriate antibodies. Only those regions of the immunoblots displaying the relevant proteins are shown. The PulK antibodies also reacted with two soluble proteins that remained at the bottom of the gradient and with an outer membrane protein (probably OmpA), which serve as markers. Cytoplasmic and outer membrane proteins were visualized by protein staining, and cytoplasmic membrane protein SecG was detected by immunoblotting. The bands designated PulS, PulK, and PulG were not detected in membranes from strains carrying pCHAP1219 (pCHAP231 pulS), pCHAP1323 (pCHAP231 pulK), and pCHAP1216 (pCHAP231 pulG) (26).
FIG. 2
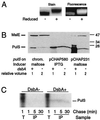
Evidence that PulS contains a DsbA-catalyzed intramolecular disulfide bond (A) Effect of reduction of purified PulS-His8 on its migration and on the accessibility of cysteine residues to fluorescein 5-maleimide. The labeled protein was separated on a 12% acrylamide gel and either stained with Coomassie blue (left panel) or photographed under UV light (right panel). (B) Examination of PulS protein encoded in wild-type and DsbA− strains of E. coli K-12 carrying two different plasmids (pCHAP580 and pCHAP231) (strains JCB570 and JCB571, respectively) or with pulS in the chromosomal gene cluster (strains PAP7232 and PAP7246, respectively). Proteins were separated on an 11% acrylamide gel and immunoblotted with antibodies against a MalE-PulS hybrid protein. Note that twice as much material was loaded from the DsbA− mutants as from the wild-type strain. MalE protein encoded by the chromosomal malE gene in strains induced with maltose (pCHAP231 and chromosomal pul genes) serves as an internal control. (C) Stability of PulS encoded by pCHAP580 in strains PAP7498 (dsbA) and PAP7460 (wild type), as determined by pulse-labeling with 14C-amino acids and a chase with unlabeled Casamino Acids. Samples of total labeled cells at 1 min after addition of chase (T) and proteins immunoprecipitated with antibodies against MalE-PulS (IP) after 1, 5, and 30 min of chase were separated on an 11.3% acrylamide–8 M urea SDS-polyacrylamide gel and detected by fluorography. The positions of prestained molecular size markers (in kilodaltons) are indicated in panel B.
FIG. 3
Processing of LacZ-PulK protein by PulO. Extracts of cells from IPTG-induced cultures of strain PAP7460 carrying pCHAP1271 (lacZ′-′pulK) with or without pCHAP576 (lacZp-pulO) were separated by SDS-PAGE on an 11.3% acrylamide–8 M urea gel and then immunoblotted with antibodies against MalE-PulK. Only that portion of the immunoblot displaying precursor (prePulK) and mature (PulK) forms of LacZ-PulK is shown.
FIG. 4
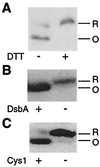
Evidence that PulK contains a DsbA-catalyzed intramolecular disulfide bond. (A) Effect of dithiothreitol (DTT) (10 mM) on migration of pCHAP1271-encoded LacZ′-′PulK hybrid protein in an 11.3% acrylamide–8M urea SDS-polyacrylamide gel. (B) Immunodetection of PulK in strains PAP7460 (DsbA+) and PAP7498 (DsbA−) carrying pCHAP1270 (lacZp-pulK). Proteins were separated in a 10% acrylamide gel. (C) Comparison of electrophoretic mobilities of PulK with (pCHAP1270) and without (pCHAP1329) Cys1 in a 10% acrylamide gel. All cultures were grown in the presence of IPTG to induce pulK. Immunoblotting with anti-MalE-PulK was performed as described for Fig. 3. Only those parts of the immunoblots displaying PulK are shown. R, reduced; O, oxidized.
FIG. 5
Effect of cysteine substitutions on immunodetection and stability of PulS determined by pulse-chase analysis of PulS variants produced by strain PAP7460 carrying pCHAP580 or its derivatives with Cys1 or Cys2 substitutions. The control plasmid (none) was the empty vector (pSU19). Proteins were labeled with 14C-amino acids and immunoprecipitated with antibodies against MalE-PulS after the indicated times of chase with unlabeled Casamino Acids.
FIG. 6
Restoration of pullulanase secretion in DsbA− strain PAP7498 by overproduction of PulK. Cells (lanes C) of PAP7460 (DsbA+) and PAP7498 carrying pCHAP710 and pCHAP4260 (lacZp-pulA) or its derivative with genes pulS (pCHAP1368) or pulK (pCHAP1367) cloned behind pulA were grown in medium containing maltose and IPTG and then centrifuged to separate than from the medium (lanes M). The 9% acrylamide gel was loaded with equal amounts (5-μl equivalent of initial culture) of the two samples. Pullulanase was detected by immunoblotting with PulA antiserum.
FIG. 7
DsbA is not needed for secreton pilus formation but is needed for production of type IV pilin PpdD in strains with the pul genes integrated into the chromosome. (A) Immunodetection of PulG in total cell extracts (lanes T) and in material released by shearing (lanes R) from plate-grown cultures of strain PAP7500(pCHAP163) or its DsbA− derivative PAP7498(pCHAP163). Proteins were separated on an 11.3% acrylamide–8 M urea gel and immunoblotted with antibodies against PulG. (B) Immunodetection of PpdD in plate-grown cultures of strain PAP7500(pCHAP3100) and its DsbA− derivative PAP7499(pCHAP3100). Samples were treated as described for panel A, and PpdD was immunodetected with antibodies against MalE-PpdD.
Similar articles
- Type IV-like pili formed by the type II secreton: specificity, composition, bundling, polar localization, and surface presentation of peptides.
Vignon G, Köhler R, Larquet E, Giroux S, Prévost MC, Roux P, Pugsley AP. Vignon G, et al. J Bacteriol. 2003 Jun;185(11):3416-28. doi: 10.1128/JB.185.11.3416-3428.2003. J Bacteriol. 2003. PMID: 12754241 Free PMC article. - Pilus formation and protein secretion by the same machinery in Escherichia coli.
Sauvonnet N, Vignon G, Pugsley AP, Gounon P. Sauvonnet N, et al. EMBO J. 2000 May 15;19(10):2221-8. doi: 10.1093/emboj/19.10.2221. EMBO J. 2000. PMID: 10811613 Free PMC article. - Multiple interactions between pullulanase secreton components involved in stabilization and cytoplasmic membrane association of PulE.
Possot OM, Vignon G, Bomchil N, Ebel F, Pugsley AP. Possot OM, et al. J Bacteriol. 2000 Apr;182(8):2142-52. doi: 10.1128/JB.182.8.2142-2152.2000. J Bacteriol. 2000. PMID: 10735856 Free PMC article.
Cited by
- The type II secretion system: biogenesis, molecular architecture and mechanism.
Korotkov KV, Sandkvist M, Hol WG. Korotkov KV, et al. Nat Rev Microbiol. 2012 Apr 2;10(5):336-51. doi: 10.1038/nrmicro2762. Nat Rev Microbiol. 2012. PMID: 22466878 Free PMC article. Review. - F-like type IV secretion systems encode proteins with thioredoxin folds that are putative DsbC homologues.
Elton TC, Holland SJ, Frost LS, Hazes B. Elton TC, et al. J Bacteriol. 2005 Dec;187(24):8267-77. doi: 10.1128/JB.187.24.8267-8277.2005. J Bacteriol. 2005. PMID: 16321931 Free PMC article. - Type IV-like pili formed by the type II secreton: specificity, composition, bundling, polar localization, and surface presentation of peptides.
Vignon G, Köhler R, Larquet E, Giroux S, Prévost MC, Roux P, Pugsley AP. Vignon G, et al. J Bacteriol. 2003 Jun;185(11):3416-28. doi: 10.1128/JB.185.11.3416-3428.2003. J Bacteriol. 2003. PMID: 12754241 Free PMC article. - Dual Role for DsbA in Attacking and Targeted Bacterial Cells during Type VI Secretion System-Mediated Competition.
Mariano G, Monlezun L, Coulthurst SJ. Mariano G, et al. Cell Rep. 2018 Jan 16;22(3):774-785. doi: 10.1016/j.celrep.2017.12.075. Cell Rep. 2018. PMID: 29346773 Free PMC article. - DsbA and DsbC are required for secretion of pertussis toxin by Bordetella pertussis.
Stenson TH, Weiss AA. Stenson TH, et al. Infect Immun. 2002 May;70(5):2297-303. doi: 10.1128/IAI.70.5.2297-2303.2002. Infect Immun. 2002. PMID: 11953363 Free PMC article.
References
- Bardwell J C A, McGovern K, Beckwith J. Identification of a protein required for disulfide bond formation in vivo. Cell. 1991;65:581–589. - PubMed
- Bartolomé B, Jubete Y, Martinez E, de la Cruz F. Construction and properties of a family of pACYC184-derved cloning vectors compatible with pBR322 and its derivatives. Gene. 1991;102:75–78. - PubMed
- Bleves S, Lazdunski A, Tommassen J, Filloux A. The secretion apparatus of Pseudomonas aeruginosa: identification of a fifth pseudopilin, XcpX. Mol Microbiol. 1998;27:31–40. - PubMed
- Bortoli-German I, Brun E, Py B, Chippaux M, Barras F. Periplasmic disulphide bond formation is essential for cellulase secretion by the plant pathogen Erwinia chrysanthemi. Mol Microbiol. 1994;11:545–553. - PubMed
- Brok R, Van Gelder P, Winterhalter M, Ziese U, Koster A J, de Cock H, Koster M, Tommassen J, Bitter W. The C-terminal domain of the Pseudomonas secretin XcpQ forms oligomeric rings with pore activity. J Mol Biol. 1999;294:1169–1179. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources