Apical membrane localization of the adenomatous polyposis coli tumor suppressor protein and subcellular distribution of the beta-catenin destruction complex in polarized epithelial cells - PubMed (original) (raw)
Apical membrane localization of the adenomatous polyposis coli tumor suppressor protein and subcellular distribution of the beta-catenin destruction complex in polarized epithelial cells
A Reinacher-Schick et al. J Cell Biol. 2001.
Abstract
The adenomatous polyposis coli (APC) protein is implicated in the majority of hereditary and sporadic colon cancers. APC is known to function as a tumor suppressor through downregulation of beta-catenin as part of a high molecular weight complex known as the beta-catenin destruction complex. The molecular composition of the intact complex and its site of action in the cell are still not well understood. Reports on the subcellular localization of APC in various cell systems have differed significantly and have been consistent with an association with a cytosolic complex, with microtubules, with the nucleus, or with the cortical actin cytoskeleton. To better understand the role of APC and the destruction complex in colorectal cancer, we have begun to characterize and isolate these complexes from confluent polarized human colon epithelial cell monolayers and other epithelial cell types. Subcellular fractionation and immunofluorescence microscopy reveal that a predominant fraction of APC associates tightly with the apical plasma membrane in a variety of epithelial cell types. This apical membrane association is not dependent on the mutational status of either APC or beta-catenin. An additional pool of APC is cytosolic and fractionates into two distinct high molecular weight complexes, 20S and 60S in size. Only the 20S fraction contains an appreciable portion of the cellular axin and small but detectable amounts of glycogen synthase kinase 3beta and beta-catenin. Therefore, it is likely to correspond to the previously characterized beta-catenin destruction complex. Dishevelled is almost entirely cytosolic, but does not significantly cofractionate with the 20S complex. The disproportionate amount of APC in the apical membrane and the lack of other destruction complex components in the 60S fraction of APC raise questions about whether these pools of APC take part in the degradation of beta-catenin, or alternatively, whether they could be involved in other functions of the protein that still must be determined.
Figures
Figure 1
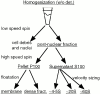
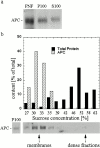
Schematic representation of fractionation protocol used to analyze subcellular distribution of APC and components of the β-catenin destruction complex in HCT116 and MCF-7 cells. Cells were lysed in hypotonic lysis buffer without detergents (w/o det.) and homogenized using a Dounce homogenizer. Unbroken cells and nuclei were removed by low speed centrifugation (5,000 rpm for 30 min). Postnuclear supernatants were further fractionated by high speed centrifugation (100,000 g for 1 h) into a pellet fraction (P100, >90S) and a supernatant fraction (S100, <90S). Membrane association of proteins was analyzed by sucrose density equilibrium flotation of P100 fractions (density of membranes ∼1.13 g/cm3; density of dense fractions ∼1.3 g/cm3). The S100 sample was further fractionated by velocity sedimentation. Size fractions of ∼4–5S, ∼20S, and ∼60S were distinguished based on distribution of total protein, APC, and axin.
Figure 5
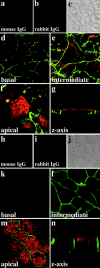
Apical localization of APC in the MCF-7 breast tumor cell line and the MDCK cell line. Immunolocalization of APC (red) and β-catenin (green) in MCF-7 (a–g) and MDCK cells (h–n). Images of successive sections of fully confluent MCF-7 and MDCK cells using confocal microscopy. (d and k) Basal; (e and l) intermediate; and (f and m) apical sections as well as (g and n) corresponding perpendicular section (z-axis) for MCF-7 and MDCK cells. Negative controls (substitution of normal mouse or rabbit IgG for primary antibodies) are shown for MCF-7 cells in a and b and for MDCK cells in h and i, respectively. (c and j) Phase–contrast image of controls.
Figure 2
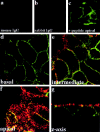
Membrane association of APC in HCT116 colon carcinoma cells. (a) Western blot showing distribution of APC after high speed centrifugation. APC present in the postnuclear fraction (PNF) distributes into both the high speed pellet fraction (P100) and the high speed supernatant fraction (S100). Equal proportions of P100 and S100 samples were loaded. (b) Dilution series for measurement of APC by Western blotting. (c) APC floats with membranes in equilibrium density gradients. Graph and corresponding Western blot illustrating distribution of APC, E-cadherin (marker for membranes), and total protein in each fraction after equilibrium density flotation of P100 fractions. (d) Solubility of membrane-bound APC after treatment with detergents or high salt. Membrane-containing fractions after P100 density flotation (fractions 30–33% in panel c) were pooled, treated with 1% NP-40, 1% octyl glucoside (OG) or 1 M NaCl, and subjected to a high speed spin. One third of starting material was loaded in lane M (membrane) and resulting pellet and supernatant fractions were loaded in lanes P and S, respectively. Membrane-bound APC is only partially solubilized by solubilization of membranes with detergents, whereas high salt treatment does not result in the release of APC into the soluble fraction. Moreover, after high salt treatment, APC continues to float with membranes in density gradients (data not shown).
Figure 3
Apical localization of APC in the HCT116 colon carcinoma cell line_._ Immunolocalization of endogenous APC (red) and β-catenin (green) in HCT116 cells. (a and b) Negative control for APC and β-catenin staining, respectively. Normal mouse (a) or normal rabbit IgG (b) was substituted for primary antibodies before incubation with respective secondary antibodies. (c) Peptide competition (+ peptide apical). Anti–β-catenin and anti-APC antibodies were preincubated with an excess of the neutralizing peptide against which the anti-APC antibody was raised. A transverse apical section is shown. (d–f) Images of successive sections of fully confluent, polarized HCT116 cells using confocal microscopy: basal (d), intermediate (e), and apical (f) sections. (g) Corresponding perpendicular section (z-axis).
Figure 4
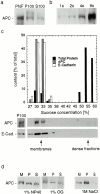
Membrane association of APC in MCF-7 breast epithelial cancer cells_._ (a) Western blot for APC illustrating distribution of APC after fractionation of postnuclear fractions (PNF) into a high speed pellet fraction (P100) and a high speed supernatant fraction (S100). Equal proportions of P100 and S100 were loaded. (b) APC in MCF-7 cells fractionates with membranes in equilibrium density gradients. Graph illustrating relative distribution of total protein and APC in each fraction after density flotation of P100 samples. Corresponding Western blot for APC is shown below.
Figure 6
Apical membrane localization of APC in normal mouse colon. Immunolocalization of APC (red) and β-catenin (green) in tissue sections of normal mouse colon. (a and b) Apical membrane staining of epithelial cells in the upper portion of colonic crypts which face the lumen of the digestive tract in normal mouse colon (arrows). (c) Low APC immunoreactivity in epithelial cells towards the base of the crypts. Additional APC staining in nonepithelial cells in the lamina propria (arrowheads) and throughout the submucosa and muscularis layers. (d) APC staining is effectively blocked with the specific neutralizing peptide against which the antibody was raised. There is no β-catenin staining, because normal mouse IgG was substituted for β-catenin primary antibody in control sections. Note residual immunoreactivity of secondary anti–mouse IgG antibody with cells in the lamina propria (green).
Figure 9
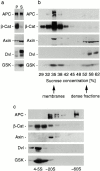
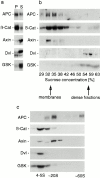
Distinct fractionation pattern of components of the β-catenin destruction complex in HCT116 cells. Cells were fractionated according to the scheme in Fig. 1 and analyzed by Western blotting for APC, β-catenin (β-Cat), axin, dishevelled (Dvl), and GSK-3β (GSK). (a) Distribution of proteins into pellet (P100) and supernatant (S100) after high speed centrifugation. Equal proportions of P100 and S100 were loaded. Note that axin, dishevelled, and GSK are mostly cytosolic. (b) Distribution of proteins of the destruction complex and β-catenin after density equilibrium flotation of P100 fractions. Note that only a small fraction of total axin, dishevelled, and GSK-3β is analyzed by P100 flotation since these components are mostly cytosolic. (c) Distribution of components of the destruction complex and β-catenin after velocity sizing of S100 fractions. APC distributes into two high molecular weight pools of ∼20S and 60S.
Figure 7
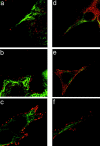
Localization of APC and microtubules in subconfluent epithelial cells. Immunolocalization of APC (red) and β-tubulin (green) in subconfluent HCT116 (a–c) and MDCK cells (d–e). (a) Localization of APC at the tips of cell processes containing microtubules is detected, albeit infrequently (<5% of all microtubule-containing protrusions). (b–f) APC is present over much of the cell surface and enriched all along the edges of the cell body and cell protrusions. (f) Higher magnification view of e.
Figure 8
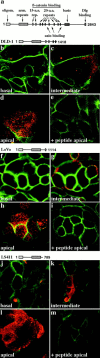
Localization of mutant forms of APC to the apical membrane in colon cancer cell lines. (a) Linear representation of the full-length human APC protein. Several known motifs are shown on top, including the oligomerization domain (oligom.), armadillo repeats (arm. repeats), the 15– and 20–amino acid repeats (both known to bind β-catenin), basic domain, and Dlg binding site. Regions for axin binding are shown below. Immunolocalization of APC (red) and β-catenin (green) in DLD-1 (b–e), LoVo (f–i), and LS411 (j–m) cells. Linear representation of truncated mutant forms of the APC protein expressed by the respective cell line is also shown. Images of successive sections of fully confluent DLD-1, LoVo, and LS411 cells using confocal microscopy. (b, f, and j) Basal; (c, g, and k) intermediate; and (d, h, and l) apical sections are shown. (e, i, and m) Peptide competition (+ peptide apical). Apical section through DLD-1 (e), LoVo (i), and LS411 cells (m) after preincubation of primary anti–β-catenin and anti-APC antibodies with the neutralizing peptide against which the anti-APC antibody was raised.
Figure 10
Distinct fractionation pattern of components of the β-catenin destruction complex in MCF-7 breast cancer cells. Cells were fractionated according to the scheme in Fig. 1 and fractions were analyzed by Western blotting for APC, β-catenin, axin, dishevelled, and GSK-3β. (a) Distribution of proteins into high speed pellet (P100) and high speed supernatant (S100) fractions. Equal proportions of the fractions were loaded. Note that axin, dishevelled, and GSK-3β are predominantly cytosolic. (b) Distribution of components of the destruction complex and β-catenin after density flotation of P100 fractions. Note that only a small fraction of total axin, dishevelled, and GSK-3β is analyzed by P100 flotation since these components are mostly cytosolic. (c) Distribution of components of the destruction complex after velocity centrifugation of S100 fractions. Similar to HCT116 cells, APC distributes into two high molecular weight peaks of ∼20S and ∼60S in MCF-7 cells.
Similar articles
- Domains of axin involved in protein-protein interactions, Wnt pathway inhibition, and intracellular localization.
Fagotto F, Jho Eh, Zeng L, Kurth T, Joos T, Kaufmann C, Costantini F. Fagotto F, et al. J Cell Biol. 1999 May 17;145(4):741-56. doi: 10.1083/jcb.145.4.741. J Cell Biol. 1999. PMID: 10330403 Free PMC article. - Downregulation of beta-catenin by human Axin and its association with the APC tumor suppressor, beta-catenin and GSK3 beta.
Hart MJ, de los Santos R, Albert IN, Rubinfeld B, Polakis P. Hart MJ, et al. Curr Biol. 1998 May 7;8(10):573-81. doi: 10.1016/s0960-9822(98)70226-x. Curr Biol. 1998. PMID: 9601641 - Modulation of Wnt signaling by Axin and Axil.
Kikuchi A. Kikuchi A. Cytokine Growth Factor Rev. 1999 Sep-Dec;10(3-4):255-65. doi: 10.1016/s1359-6101(99)00017-9. Cytokine Growth Factor Rev. 1999. PMID: 10647780 Review. - Signaling through beta-catenin and Lef/Tcf.
Novak A, Dedhar S. Novak A, et al. Cell Mol Life Sci. 1999 Oct 30;56(5-6):523-37. doi: 10.1007/s000180050449. Cell Mol Life Sci. 1999. PMID: 11212302 Free PMC article. Review.
Cited by
- Actin-dependent membrane association of the APC tumour suppressor in polarized mammalian epithelial cells.
Rosin-Arbesfeld R, Ihrke G, Bienz M. Rosin-Arbesfeld R, et al. EMBO J. 2001 Nov 1;20(21):5929-39. doi: 10.1093/emboj/20.21.5929. EMBO J. 2001. PMID: 11689433 Free PMC article. - Colocalization of APC and DLG at the tips of cellular protrusions in cultured epithelial cells and its dependency on cytoskeletons.
Iizuka-Kogo A, Shimomura A, Senda T. Iizuka-Kogo A, et al. Histochem Cell Biol. 2005 Jan;123(1):67-73. doi: 10.1007/s00418-004-0729-2. Epub 2004 Dec 18. Histochem Cell Biol. 2005. PMID: 15609045 - Regulation of retromer recruitment to endosomes by sequential action of Rab5 and Rab7.
Rojas R, van Vlijmen T, Mardones GA, Prabhu Y, Rojas AL, Mohammed S, Heck AJ, Raposo G, van der Sluijs P, Bonifacino JS. Rojas R, et al. J Cell Biol. 2008 Nov 3;183(3):513-26. doi: 10.1083/jcb.200804048. J Cell Biol. 2008. PMID: 18981234 Free PMC article.
References
- Boutros M., Paricio N., Strutt D.I., Mlodzik M. Dishevelled activates JNK and discriminates between JNK pathways in planar polarity and wingless signaling. Cell. 1998;94:109–118. - PubMed
- Guger K.A., Gumbiner B.M. A mode of regulation of beta-catenin signaling activity in Xenopus embryos independent of its levels. Dev. Biol. 2000;223:441–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 GM037432/GM/NIGMS NIH HHS/United States
- R37 GM037432/GM/NIGMS NIH HHS/United States
- NCI-P30-CA-08784/CI/NCPDCID CDC HHS/United States
- GM37432/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous