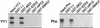The polycomb group protein EED interacts with YY1, and both proteins induce neural tissue in Xenopus embryos - PubMed (original) (raw)
The polycomb group protein EED interacts with YY1, and both proteins induce neural tissue in Xenopus embryos
D P Satijn et al. Mol Cell Biol. 2001 Feb.
Abstract
Polycomb group (PcG) proteins form multimeric protein complexes which are involved in the heritable stable repression of genes. Previously, we identified two distinct human PcG protein complexes. The EED-EZH protein complex contains the EED and EZH2 PcG proteins, and the HPC-HPH PcG complex contains the HPC, HPH, BMI1, and RING1 PcG proteins. Here we show that YY1, a homolog of the Drosophila PcG protein pleiohomeotic (Pho), interacts specificially with the human PcG protein EED but not with proteins of the HPC-HPH PcG complex. Since YY1 and Pho are DNA-binding proteins, the interaction between YY1 and EED provides a direct link between the chromatin-associated EED-EZH PcG complex and the DNA of target genes. To study the functional significance of the interaction, we expressed the Xenopus homologs of EED and YY1 in Xenopus embryos. Both Xeed and XYY1 induce an ectopic neural axis but do not induce mesodermal tissues. In contrast, members of the HPC-HPH PcG complex do not induce neural tissue. The exclusive, direct neuralizing activity of both the Xeed and XYY1 proteins underlines the significance of the interaction between the two proteins. Our data also indicate a role for chromatin-associated proteins, such as PcG proteins, in Xenopus neural induction.
Figures
FIG. 1
Interaction between the EED and YY1 proteins. (A) Two-hybrid analysis with YY1 and the indicated vertebrate PcG proteins showed a positive interaction between YY1 and EED. The PcG cDNAs were cloned in frame with the GAL4 DNA-binding domain (DBD), and the YY1 cDNA was cloned in frame with the GAL4 transactivation domain (AD). No interactions between the PcG proteins and simian virus 40 were observed. (B) The domain of YY1 that interacted with EED was mapped. Indicated portions of YY1 were fused to the GAL4 AD. β-Gal, β-galactosidase. (C) Deletion of the most N-terminal WD-40 domain in EED resulted in abolishment of the interaction between YY1 and EED.
FIG. 2
In vivo interaction between EED and YY1 or Pho. Extracts from human Ramos cells were immunoprecipitated using antibodies against EED, EZH2, YY1, or HPC2 or without antibody (mock IP). Western blots of the IPs were probed with antibodies against EED, EZH2, YY1, or HPC2. No antigens were detected when the specific IP antibodies were omitted from the IPs (mock IP). Input, extract from Ramos cells.
FIG. 3
In vitro interaction between EED and YY1 or Pho. In vitro-translated, [35S]methionine-labeled YY1 or Pho was incubated with immobilized GST [GST (−)], GST-EED, GST-EZH2, GST-HPC2, or GST-RING1. The input was 15% of the amount that was incubated with the GST fusion proteins.
FIG. 4
Predicted amino acid sequence of Xeed. The Xeed protein was compared with the human EED protein. Identical amino acids are shaded. Five WD-40 repeats are indicated with boxes.
FIG. 5
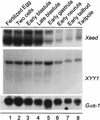
Developmental expression profiles of Xeed and XYY1. Probes for the Xeed and XYY1 genes were used for Northern analysis of total RNA (20 μg) isolated from the indicated developmental stages (15). The filter was rehybridized with a probe for Gαs-1 (19) to verify the loading and integrity of RNA in each lane.
FIG. 6
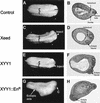
Phenotypes resulting from injection of Xeed, XYY1, and XYY1-EnR mRNA into Xenopus embryos. (A, C, E, and G) Indicated mRNAs were injected into one blastomere of two-cell-stage Xenopus embryos. The side of the embryo that was injected is indicated. The broadening of the neural plate in Xeed-injected (C) and XYY1-injected (E) embryos, compared to the uninjected embryo (A), is indicated with brackets. The ectopic axis in XYY1-EnR-injected embryos (G) is also indicated, as is the cement gland (C). (B, D, F, and H) The embryos in panels A, C, E, and G were processed for histological analysis in order to visualize the somites, notochord, and neural tissue. Shown are histological sections of uninjected (B), Xeed-injected (D), XYY1-injected (F), and XYY1-EnR-injected (H) embryos.
FIG. 7
Lack of phenotypes from injection of XPc and mutants of Xeed and XYY1. Indicated mRNAs were injected into one blastomere of two-cell-stage Xenopus embryos. The side of the embryo that was injected is indicated. Injection of neither XPc (A and B), Xeed (aa 1 to 385) (C and D), nor XYY1 (aa 1 to 250) (D and E) induced broadening of the neural plate or histologically defined neural tissue.
FIG. 8
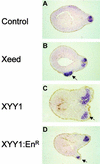
Induction of neural tissue in Xeed- and XYY1-injected embryos. Xeed (B), XYY1 (C), and XYY1-EnR (D) mRNAs were injected into one blastomere of the two-cell-stage Xenopus embryo and cultured until stage 23. All injected embryos, as well as the uninjected control embryos (A), were subjected to whole-mount in situ hybridization using a probe that detects the neural marker NRP-1. Subsequently, the embryos were processed for histological analysis and sections were examined. The ectopic neural tissue in Xeed-, XYY1-, and XYY1-EnR-injected embryos is indicated with arrows.
FIG. 9
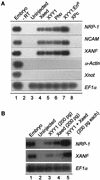
Direct neural induction in ectoderm by Xeed and XYY1. (A) Two nanograms of Xeed, XYY1, or Pho mRNA, 100 pg of XYY1-EnR mRNA, and 2 ng of XPc mRNA were injected into two blastomeres of two-cell Xenopus embryos and cultured to stage 10 (early gastrula). The entire ectoderm was dissected and cultured to stage 25, when RNA was isolated and analyzed by RT-PCR using primers recognizing the neural markers NRP-1, NCAM, and XANF as well as the mesodermal marker α-actin and the notochord marker Xnot. As a control, dissected stage 10 ectoderm of an uninjected embryo (lane 3) was cultured to stage 25. Uninjected, whole stage 23 embryos were analyzed as a positive control (lane 1). To verify the presence of equal amounts of RNA, EF1α was used as loading marker. −RT, no reverse transcriptase was added. (B) Two hundred picograms of Xeed (lane 3), 200 pg of XYY1 (lane 4), or 200 pg of Xeed plus 200 pg of XYY1 (lane 5) were injected into two blastomeres of two-cell Xenopus embryos and the experiment proceeded as for panel A.
FIG. 10
Composition of human PcG protein complexes. The HPC-HPH PcG complex contains the PcG proteins HPC2, BMI1, HPH1, HPH2, and RING1A. The EED-EZH PcG complex contains the PcG proteins EED and EZH2 and is associated with HDAC proteins. In this article we show that the homolog of the Drosophila PcG protein Pho, YY1, interacts with the EED protein and thus is either part of or associated with the EED-EZH PcG complex.
Similar articles
- Transcriptional repression mediated by the human polycomb-group protein EED involves histone deacetylation.
van der Vlag J, Otte AP. van der Vlag J, et al. Nat Genet. 1999 Dec;23(4):474-8. doi: 10.1038/70602. Nat Genet. 1999. PMID: 10581039 - The Drosophila Polycomb group gene pleiohomeotic encodes a DNA binding protein with homology to the transcription factor YY1.
Brown JL, Mucci D, Whiteley M, Dirksen ML, Kassis JA. Brown JL, et al. Mol Cell. 1998 Jun;1(7):1057-64. doi: 10.1016/s1097-2765(00)80106-9. Mol Cell. 1998. PMID: 9651589 - Transcription factor YY1 functions as a PcG protein in vivo.
Atchison L, Ghias A, Wilkinson F, Bonini N, Atchison ML. Atchison L, et al. EMBO J. 2003 Mar 17;22(6):1347-58. doi: 10.1093/emboj/cdg124. EMBO J. 2003. PMID: 12628927 Free PMC article. - Gene repression by Polycomb group protein complexes: a distinct complex for every occasion?
Otte AP, Kwaks TH. Otte AP, et al. Curr Opin Genet Dev. 2003 Oct;13(5):448-54. doi: 10.1016/s0959-437x(03)00108-4. Curr Opin Genet Dev. 2003. PMID: 14550408 Review. - Polycomb-group genes and hematopoiesis.
Takihara Y, Hara J. Takihara Y, et al. Int J Hematol. 2000 Aug;72(2):165-72. Int J Hematol. 2000. PMID: 11039664 Review.
Cited by
- Polycomb group gene rae28 is required for sustaining activity of hematopoietic stem cells.
Ohta H, Sawada A, Kim JY, Tokimasa S, Nishiguchi S, Humphries RK, Hara J, Takihara Y. Ohta H, et al. J Exp Med. 2002 Mar 18;195(6):759-70. doi: 10.1084/jem.20011911. J Exp Med. 2002. PMID: 11901201 Free PMC article. - Zinc Ions Modulate YY1 Activity: Relevance in Carcinogenesis.
Figiel M, Górka AK, Górecki A. Figiel M, et al. Cancers (Basel). 2023 Aug 30;15(17):4338. doi: 10.3390/cancers15174338. Cancers (Basel). 2023. PMID: 37686614 Free PMC article. Review. - YY1 knockout in pro-B cells impairs lineage commitment, enabling unusual hematopoietic lineage plasticity.
Banerjee S, Sanyal S, Hodawadekar S, Naiyer S, Bano N, Banerjee A, Rhoades J, Dong D, Allman D, Atchison ML. Banerjee S, et al. Genes Dev. 2024 Oct 16;38(17-20):887-914. doi: 10.1101/gad.351734.124. Genes Dev. 2024. PMID: 39362773 Free PMC article. - Acetylated YY1 regulates Otx2 expression in anterior neuroectoderm at two cis-sites 90 kb apart.
Takasaki N, Kurokawa D, Nakayama R, Nakayama J, Aizawa S. Takasaki N, et al. EMBO J. 2007 Mar 21;26(6):1649-59. doi: 10.1038/sj.emboj.7601619. Epub 2007 Mar 1. EMBO J. 2007. PMID: 17332747 Free PMC article. - Polycomb recruitment to DNA in vivo by the YY1 REPO domain.
Wilkinson FH, Park K, Atchison ML. Wilkinson FH, et al. Proc Natl Acad Sci U S A. 2006 Dec 19;103(51):19296-301. doi: 10.1073/pnas.0603564103. Epub 2006 Dec 8. Proc Natl Acad Sci U S A. 2006. PMID: 17158804 Free PMC article.
References
- Brown J L, Mucci D, Whiteley M, Dirksen M L, Kassis J A. The Drosophila Polycomb group gene pleiohomeotic encodes a sequence-specific DNA binding protein with homology to the multifunctional mammalian transcription factor YY1. Mol Cell. 1998;7:1057–1064. - PubMed
- Conlon F L, Sedgwick S G, Weston K M, Smith J C. Inhibition of Xbra transcription activation causes defects in mesodermal patterning and reveals autoregulation of Xbra in dorsal mesoderm. Development. 1996;122:2427–2435. - PubMed
- Gould A. Functions of mammalian Polycomb group and trithorax group related genes. Curr Opin Genet Dev. 1997;7:488–494. - PubMed
- Gunster J M, Satijn D P E, Hamer K M, den Blaauwen J L, de Bruijn D, Alkema M J, van Lohuizen M, van Driel R, Otte A P. Identification and characterization of interactions between the vertebrate Polycomb-group protein BMI1 and human homologs of Polyhomeotic. Mol Cell Biol. 1997;17:2326–2335. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases