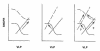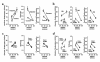Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes - PubMed (original) (raw)
Comment
Ornithine decarboxylase, kidney size, and the tubular hypothesis of glomerular hyperfiltration in experimental diabetes
S C Thomson et al. J Clin Invest. 2001 Jan.
Abstract
In early diabetes, the kidney grows and the glomerular filtration rate (GFR) increases. This growth is linked to ornithine decarboxylase (ODC). The study of hyperfiltration has focused on microvascular abnormalities, but hyperfiltration may actually result from a prior increase in capacity for proximal reabsorption which reduces the signal for tubuloglomerular feedback (TGF). Experiments were performed in Wistar rats after 1 week of streptozotocin diabetes. Kidney weight, ODC activity, and GFR were correlated in diabetic and control rats given difluoromethylornithine (DFMO; Marion Merrell Dow, Cincinnati, Ohio, USA) to inhibit ODC. We assessed proximal reabsorption by micropuncture, using TGF as a tool for manipulating single-nephron GFR (SNGFR), then plotting proximal reabsorption versus SNGFR. ODC activity was elevated 15-fold in diabetic kidneys and normalized by DFMO, which also attenuated hyperfiltration and hypertrophy. Micropuncture data revealed an overall increase in proximal reabsorption in diabetic rats too great to be accounted for by glomerulotubular balance. DFMO prevented the overall increase in proximal reabsorption. These data confirm that ODC is required for the full effect of diabetes on kidney size and proximal reabsorption in early streptozotocin diabetes and are consistent with the hypothesis that diabetic hyperfiltration results from normal physiologic actions of TGF operating in a larger kidney, independent of any primary malfunction of the glomerular microvasculature.
Figures
Figure 1
Framework for interactions between glomerulus and proximal tubule and pathways to hyperfiltration. VLP, late proximal flow. SNGFR and VLP are linked by TGF (sigmoid curves) and GTB (straight lines) which intersect at a single operating point. The operating point cannot change unless there is a change in TGF or GTB. Hyperfiltration can result from a shift in TGF (left panel) or an increase in proximal reabsorption (center panel). A primary increase in proximal reabsorption can induce TGF to reset thus obscuring increased reabsorption as the primary cause of hyperfiltration (right panel).
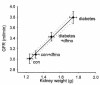
Figure 2
GFR vs. kidney weight in diabetic and control rats ± treatment with DFMO. GFR is for two kidneys. Kidney weight is wet-weight of left kidney. Dashed lines are 95% confidence intervals for linear regression. _r_2 = 0.996.
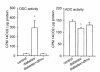
Figure 3
Activities of ODC and ADC in control and diabetic rats. A_P_ < 0.05 vs. control.
Figure 4
Pairwise intergroup comparisons of different indices of proximal reabsorption, each shown as a function of SNGFR. Line segments connect data obtained at minimum (higher SNGFR) and maximum (lower SNGFR) TGF activation. Group mean ± SEM is also shown in Table 2. Statistical significance of intergroup differences are described in the text for the regions of overlapping SNGFR. (a) Effects of diabetes. (b) Effects of DFMO in diabetes. (c) Effects of DFMO in controls. (d) Diabetes + DFMO vs. control + DFMO. DM, diabetes mellitus; Con, control.
Figure 5
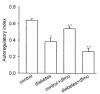
Autoregulation of tubular reabsorption by GTB. Refer to text for formula of index. Index is unity when fractional reabsorption is constant across SNGFR. Index is zero when net reabsorption is constant across SNGFR. A_P_ < 0.05 vs. control. B_P_ < 0.05 vs. diabetes.
Figure 6
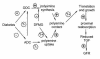
A working model to account for the effects of DFMO in the present study. The effects denoted with question marks have been demonstrated in cell culture (see text for references), but remain to be confirmed in diabetic animals.
Comment on
- Hypertrophy and hyperfunction of the diabetic kidney.
Hostetter TH. Hostetter TH. J Clin Invest. 2001 Jan;107(2):161-2. doi: 10.1172/JCI12066. J Clin Invest. 2001. PMID: 11160131 Free PMC article. No abstract available.
Similar articles
- Ornithine decarboxylase inhibitor eliminates hyperresponsiveness of the early diabetic proximal tubule to dietary salt.
Miracle CM, Rieg T, Mansoury H, Vallon V, Thomson SC. Miracle CM, et al. Am J Physiol Renal Physiol. 2008 Oct;295(4):F995-F1002. doi: 10.1152/ajprenal.00491.2007. Epub 2008 Jun 18. Am J Physiol Renal Physiol. 2008. PMID: 18562630 Free PMC article. - Inhibition of renal ornithine decarboxylase activity prevents kidney hypertrophy in experimental diabetes.
Pedersen SB, Flyvbjerg A, Richelsen B. Pedersen SB, et al. Am J Physiol. 1993 Feb;264(2 Pt 1):C453-6. doi: 10.1152/ajpcell.1993.264.2.C453. Am J Physiol. 1993. PMID: 8447376 - Inhibition of renal ornithine decarboxylase activity fails to reduce kidney size and urinary albumin excretion in diabetic rats with manifest kidney hypertrophy.
Pedersen SB, Bjørn SF, Richelsen B, Flyvbjerg A. Pedersen SB, et al. Mol Cell Endocrinol. 1995 Jan;107(1):123-8. doi: 10.1016/0303-7207(94)03433-t. Mol Cell Endocrinol. 1995. PMID: 7796931 - Sodium/glucose cotransporter 2 inhibitors and prevention of diabetic nephropathy: targeting the renal tubule in diabetes.
De Nicola L, Gabbai FB, Liberti ME, Sagliocca A, Conte G, Minutolo R. De Nicola L, et al. Am J Kidney Dis. 2014 Jul;64(1):16-24. doi: 10.1053/j.ajkd.2014.02.010. Epub 2014 Mar 25. Am J Kidney Dis. 2014. PMID: 24673844 Review. - Glomerular hemodynamic and structural alterations in experimental diabetes mellitus.
O'Donnell MP, Kasiske BL, Keane WF. O'Donnell MP, et al. FASEB J. 1988 May;2(8):2339-47. doi: 10.1096/fasebj.2.8.3282959. FASEB J. 1988. PMID: 3282959 Review.
Cited by
- Nutrient sensing, autophagy, and diabetic nephropathy.
Kume S, Thomas MC, Koya D. Kume S, et al. Diabetes. 2012 Jan;61(1):23-9. doi: 10.2337/db11-0555. Diabetes. 2012. PMID: 22187371 Free PMC article. Review. No abstract available. - Development of SGLT1 and SGLT2 inhibitors.
Rieg T, Vallon V. Rieg T, et al. Diabetologia. 2018 Oct;61(10):2079-2086. doi: 10.1007/s00125-018-4654-7. Epub 2018 Aug 22. Diabetologia. 2018. PMID: 30132033 Free PMC article. Review. - The clinical significance of hyperfiltration in diabetes.
Jerums G, Premaratne E, Panagiotopoulos S, MacIsaac RJ. Jerums G, et al. Diabetologia. 2010 Oct;53(10):2093-104. doi: 10.1007/s00125-010-1794-9. Epub 2010 May 23. Diabetologia. 2010. PMID: 20496053 Review. - Proximal tubular hypertrophy and enlarged glomerular and proximal tubular urinary space in obese subjects with proteinuria.
Tobar A, Ori Y, Benchetrit S, Milo G, Herman-Edelstein M, Zingerman B, Lev N, Gafter U, Chagnac A. Tobar A, et al. PLoS One. 2013 Sep 25;8(9):e75547. doi: 10.1371/journal.pone.0075547. eCollection 2013. PLoS One. 2013. PMID: 24086563 Free PMC article. - Micropuncturing the nephron.
Vallon V. Vallon V. Pflugers Arch. 2009 May;458(1):189-201. doi: 10.1007/s00424-008-0581-7. Epub 2008 Aug 28. Pflugers Arch. 2009. PMID: 18752000 Free PMC article. Review.
References
- O’Bryan GT, Hostetter TH. The renal hemodynamic basis of diabetic nephropathy. Semin Nephrol. 1997;17:93–100. - PubMed
- Parving, H.H., Osterby, R., and Ritz, E. 2000. Diabetic nephropathy. In The kidney. 6th edition. B. Brenner, editor. WB Saunders Co. Philadelphia, Pennsylvania, USA. 1731–1773.
- Daniels FH, Arendshorst WJ. Tubuloglomerular feedback kinetics in spontaneously hypertensive and Wistar-Kyoto rats. Am J Physiol. 1990;259:F529–F534. - PubMed
- Mogensen CE, Andersen MJF. Increased kidney size and glomerular filtration rate in early juvenile diabetes. Diabetes. 1973;22:706–712. - PubMed
- Ross J, Goldman JK. Effect of streptozotocin-induced diabetes on kidney weight and compensatory hypertrophy in the rat. Endocrinology. 1971;88:1079–1083. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials