Bmp4 mediates apoptotic cell death in the developing chick eye - PubMed (original) (raw)
Comparative Study
Bmp4 mediates apoptotic cell death in the developing chick eye
F Trousse et al. J Neurosci. 2001.
Abstract
The bone morphogenetic protein (BMP) expression in vertebrates suggests a reiterative function of these molecules during eye development. However, genetic analysis in mice has provided only partial information. Using the chick embryo as a model system, we have analyzed possible additional functions of BMP4 during optic cup formation. Here we describe the expression pattern of Bmp4 and Bmp7 and we show that, in contrast to the mouse, the prospective lens placode ectoderm expresses high levels of Bmp4 but no Bmp7. After optic vesicle invagination, Bmp4 is expressed in the prospective dorsal neural retina, where BmprIA, BmprII, and Smad1, components of the BMP4 signal transduction pathway, are also expressed. In toto terminal deoxynucleotidyl transferase-mediated biotinylated UTP nick end-labeling analysis shows that the dorsal optic cup is the site of a spatiotemporally restricted apoptosis, which parallels the expression not only of Bmp4 but also of Msx1 and Msx2, genes implicated in BMP4-mediated apoptosis. The use of optic vesicle cultures as well as in ovo local addition of BMP4 and its antagonist Noggin proves that the local activity of BMP4 is responsible for programmed cell death in the dorsal optic cup. In addition, we show that Noggin is able to reduce the rate of cell proliferation in the dorsal part of the optic cup whereas BMP4 increases the number of BrdU-positive cells in retina cultures. These results provide evidence that BMP4 contributes to eye development by promoting cell proliferation and programmed cell death.
Figures
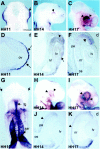
Fig. 1.
Expression pattern of Bmp4 and_Bmp7_ in the developing chick optic cup. Embryos of different developmental stages (as indicated in each_panel_) were hybridized in toto_with a digoxigenin-labeled probe against Bmp4(A–F) and Bmp7(G–K). Embryos are viewed dorsally (A), ventrally (G), or laterally (B, C, H, I). Cryostat (E, J) and paraffin (D, F, K) sections are in the frontal (E, F, J, K) or transversal (D) plane. A–F, Note the strong expression of Bmp4 in the region of the newly formed optic vesicle (A, arrow), limited only to the ectoderm (D, arrows). At the stage of the optic cup, the increasingly strong expression of_Bmp4 is limited to the dorsal portion (B, C, arrowheads), localized to the neural retina (E, F, arrowheads). Low levels of expression are present in the lens vesicle (E,arrow). G–K, Note how_Bmp7_ transcripts were totally absent from the optic vesicles (G, arrows) while strongly expressed in other regions of the embryo. Bmp7 mRNAs were first detected in the eye region at the optic cup stage (H, arrowheads) with progressively higher levels of expression (I, arrowheads). Note how transcripts are localized only to the developing pigment epithelium (J, K, arrowheads).d, Dorsal; fg, foregut region;lv, lens vesicle; nr, neural retina;os, optic stalk; ov, optic vesicle;pe, pigment epithelium; sar, sinoatrial region. Scale bar: A–C, H, I, 220 μm;G, 150 μm; D, E, F , J, K, 50 μm.
Fig. 2.
Comparative localization of PCD during chick and mouse optic cup development. Chick embryos of stage HH14 (A), HH17 (B), and HH21 (C) and mouse embryos at E9.5 (D), E10 (E), and E11 (F) were stained in toto using the TUNEL assay. Frontal cryostat sections of embryos in A, B, D, and E are illustrated in _G, I_and J, H, and K and L, respectively. The section in H is not meant to illustrate apoptosis in the lens tissue. All images are oriented with dorsal on the top. Observe that in both species there is a similar spatiotemporal distribution of TUNEL-positive nuclei in the optic stalk (compare A, G with D, H,thin arrows) and in the central region of the retina (J, L, thick arrows). Note the extensive and prolonged apoptosis in the murine lens tissue (D, E, L, thick arrows) compared with the more limited one in chick (B, I, thick arrows). Further note the high concentration of TUNEL-positive nuclei in the dorsal portion of the chick optic cup (B, I,arrowheads). Few apoptotic nuclei are observed at the equivalent stage and position in the mouse (E, K,arrowheads). Asterisks in A, D, E, and L indicate apoptotic nuclei in the mesenchyme surrounding the developing mouse eye. Thick arrow in C points to apoptotic nuclei in the lens vesicle. Dorsal; lv, lens vesicle;nr, neural retina; os, optic stalk;pe, pigment epithelium. Scale bar: A, 35 μm; B, 50 μm; C, 60 μm;D, 45 μm; E, K, L, 30 μm;F, 40 μm; G, 70 μm; H, 25 μm; I, J, 80 μm.
Fig. 3.
Components of the BMP4 signal transduction pathway are expressed in the optic cup. Lateral views are shown of HH17 (A–E, G, H) and HH21 (F) embryos hybridized in toto with digoxigenin-labeled probes for the chick BmprIA(A), BmprIB(B), BmprII(C), Noggin(D), Smad1 (E, F), Msx1 (G), and_Msx2_ (H). Note the comparatively higher levels of expression of both Noggin_and Smad1 in the dorsal optic cup (D, E,arrowheads) and the extended expression of_Smad1 at later stages (F). Note also the higher expression of BmprIB in the optic stalk (arrowheads in B). The arrow in_D_ indicates expression in the lens vesicle. In the optic cup Msx1 is expressed only dorsally (G,arrowhead), in a region contained within the domain of_Msx2_ expression (H,arrowhead). Msx2 transcripts are abundant also in the retro-ocular mesenchyme. Scale bar:A–H, 345 μm.
Fig. 4.
RT-PCR amplifications of_BmprIA_, BmprIB, BmprII, and Noggin. Amplifications were performed with specific primers on 1 μl of cDNA prepared from mRNA from prospective neural retina (NR), pigment epithelium with mesenchyme (PE), and lens (L) of HH17 chick embryos. Amplifications were independently performed three times with similar results. Observe that BmprIA and_BmprII_ mRNAs were amplified from the three components of optic cup analyzed, whereas BmprIB transcripts appeared particularly abundant in the pigment epithelium.Noggin was amplified in the three tissues but with higher levels in the pigment epithelium. _GADPH_amplifications are shown for comparison. Identity of the amplified bands was confirmed by automated DNA sequencing.
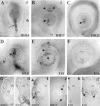
Fig. 5.
Apoptotic cell death in the developing optic is regulated by BMP4 in vitro and in vivo.A–K, The results obtained in experiments in vitro are illustrated. L–O, The effects of_in ovo_ exogenous application of noggin and BMP4 are illustrated. A, Schematic representation of the design of the optic vesicle culture is shown. The optic vesicles were dissected from stage 11 chick embryos and embedded in collagen gel matrices with the prospective lens placode upward. B–K, The vesicles were cultured for 48 hr in the absence (control culture,B) or presence of BMP4 (C), BMP7 (D), or both (E). In some cases, Noggin (Nog; F) or BMP-blocking antibody (α_BMP_;G) was incubated with the vesicles before the addition of the cytokines. The extent of apoptotic cell death was determined by NBS staining. Both BMP4 and BMP7 increased the amount of blue-stained cells (arrowheads) in the optic vesicles as well as the size of the lens vesicles as compared with control cultures (compare B with C, D). No synergistic effect was observed when both cytokines were added together (E). Note that both Noggin and anti-BMP7 antibody prevented the apoptotic cell death induced by BMP4 (F, G). TUNEL staining on paraffin sections of the cultured vesicles further confirms the increased cell death induced by BMP4 (I) when compared with control cultures (H). Immunostaining (K, J) with an antiserum against the lens-specific δ-crystallin protein verified the identity of lens tissue. Observe the increased size of the lens tissue in BMP4-treated (K) versus control (J) cultures. L–O, Ectopic Noggin represses programmed cell death in the dorsal portion of the chick optic cup. Acrylic-heparin beads containing either Noggin (M), BSA (N), or BMP4 (O) were implanted in the dorsal retro-ocular mesenchyme of HH14 embryos_in ovo_. The contralateral left eyes were used as the control (L). All images are oriented with dorsal on the top. At HH17, embryos were fixed and stained_in toto_ with TUNEL. Note that Noggin totally abolishes programmed cell death in the dorsal portion of the optic cup (L, M, arrowheads) but not in the lens vesicle (L, M, arrows). In contrast, addition of exogenous BMP4 increased the extent of TUNEL-positive nuclei normally detected in the optic cup (note the position of the_arrowheads_ in O compared with those in_L_ and N). Beads containing BSA had no effect on the extent or distribution of programmed cell death in the optic cup (N). The bead position is indicated with an asterisk. ect, Ectoderm;lv, lens vesicle; mes, mesoderm;nt, neural tube; ov, optic vesicle. Scale bar: B, 245 μm; C, E, 230 μm;D, 310 μm; F, 160 μm;G, 130 μm; H–K, 170 μm;L–O, 45 μm.
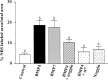
Fig. 6.
Statistical analysis of apoptotic cell death in control and treated optic vesicle cultures. Intensity of the NBS staining was quantitatively assessed on samples of scanned images representative of each experimental condition. Using an image analysis software (Q500 MC; Leica), the total and stained areas of each vesicle were measured, and the labeling intensity was calculated as the relative surface occupied by the staining. Results are expressed as means ± SEM. Letters (a, b) have been assigned to groups whose values are significantly different (a, p < 0.05; b,p < 0.001; Tukey's test). Data were analyzed by one-way ANOVA using the PRISM3 program for IBM. Note how BMP4 (n = 12) and BMP7 (n = 13) increased the extent of apoptosis in the optic vesicle in comparison with the control (n = 22; p < 0.001, Tukey's test). The effect of BMP4 is significantly reduced in the presence of Noggin (n = 21) or of anti-BMP7 antibody (α7; n = 10; p < 0.05 and p < 0.01, respectively).
Fig. 7.
Long-term addition of Noggin reduces eye size and cell proliferation in the dorsal portion of the chick optic cup. Acrylic-heparin beads containing either Noggin (B, F) or BSA (D, H) were implanted in the dorsal retro-ocular mesenchyme of the right eye of HH14 embryos_in ovo_. Embryos were analyzed 48 hr after bead implantation (HH23). A–D, In toto views of the right, manipulated (B, D) and contralateral, left (A, C) eyes of the treated embryos are shown.E–H, Frontal cryostat sections are shown of the eyes depicted in A–D, respectively. Sections are immunostained with antibody against phosphohistone H3. Note how a long-term exposure to Noggin reduces eye size (B) with respect to contralateral eyes (A, C) or to eyes exposed to BSA (D). Observe that the smaller eye size in Noggin-exposed animals is reflected in a reduction of the neural retina thickness (F) as compared with the contralateral retina (E). This reduction (E–H, arrows indicate retina thickness) was never observed in BSA-exposed (H) and the corresponding contralateral (G) retinas. The decrease in retina thickness is paralleled by a significant reduction in the number of H3-positive cells (compare staining in F with that in E, G, H; asterisk in F marks the Noggin-soaked bead). I, Quantification of the number of mitotic cells in treated and control retinas is shown. Values were obtained by counting the number of H3-positive cells in the entire dorsal portion of the right eye (black bars) of experimental (Noggin; n = 5) and control (BSA;n = 5) embryos. Values were compared with those obtained in similar counts of corresponding contralateral eyes (white bars). Note how Noggin-impregnated beads caused a significant reduction (asterisk, p< 0.01, Tukey's test) in the number of H3-positive cells. Scale bar:A, 120 μm; B, 110 μm; C, D, 130 μm; E–H, 60 μm.
Similar articles
- The level of BMP4 signaling is critical for the regulation of distinct T-box gene expression domains and growth along the dorso-ventral axis of the optic cup.
Behesti H, Holt JK, Sowden JC. Behesti H, et al. BMC Dev Biol. 2006 Dec 15;6:62. doi: 10.1186/1471-213X-6-62. BMC Dev Biol. 2006. PMID: 17173667 Free PMC article. - Microphthalmia resulting from MSX2-induced apoptosis in the optic vesicle.
Wu LY, Li M, Hinton DR, Guo L, Jiang S, Wang JT, Zeng A, Xie JB, Snead M, Shuler C, Maxson RE Jr, Liu YH. Wu LY, et al. Invest Ophthalmol Vis Sci. 2003 Jun;44(6):2404-12. doi: 10.1167/iovs.02-0317. Invest Ophthalmol Vis Sci. 2003. PMID: 12766037 - Negative and positive auto-regulation of BMP expression in early eye development.
Huang J, Liu Y, Filas B, Gunhaga L, Beebe DC. Huang J, et al. Dev Biol. 2015 Nov 15;407(2):256-64. doi: 10.1016/j.ydbio.2015.09.009. Epub 2015 Sep 25. Dev Biol. 2015. PMID: 26407529 Free PMC article. - BMP-signaling regulates the generation of hair-cells.
Pujades C, Kamaid A, Alsina B, Giraldez F. Pujades C, et al. Dev Biol. 2006 Apr 1;292(1):55-67. doi: 10.1016/j.ydbio.2006.01.001. Epub 2006 Feb 3. Dev Biol. 2006. PMID: 16458882 - Eye development and retinogenesis.
Heavner W, Pevny L. Heavner W, et al. Cold Spring Harb Perspect Biol. 2012 Dec 1;4(12):a008391. doi: 10.1101/cshperspect.a008391. Cold Spring Harb Perspect Biol. 2012. PMID: 23071378 Free PMC article. Review.
Cited by
- Analysis of Programmed Cell Death and Senescence Markers in the Developing Retina of an Altricial Bird Species.
Álvarez-Hernán G, de Mera-Rodríguez JA, Hernández-Núñez I, Marzal A, Gañán Y, Martín-Partido G, Rodríguez-León J, Francisco-Morcillo J. Álvarez-Hernán G, et al. Cells. 2021 Feb 26;10(3):504. doi: 10.3390/cells10030504. Cells. 2021. PMID: 33652964 Free PMC article. - Bone morphogenetic protein 4 mediates apoptosis of capillary endothelial cells during rat pupillary membrane regression.
Kiyono M, Shibuya M. Kiyono M, et al. Mol Cell Biol. 2003 Jul;23(13):4627-36. doi: 10.1128/MCB.23.13.4627-4636.2003. Mol Cell Biol. 2003. PMID: 12808102 Free PMC article. - BMP signaling mediates stem/progenitor cell-induced retina regeneration.
Haynes T, Gutierrez C, Aycinena JC, Tsonis PA, Del Rio-Tsonis K. Haynes T, et al. Proc Natl Acad Sci U S A. 2007 Dec 18;104(51):20380-5. doi: 10.1073/pnas.0708202104. Proc Natl Acad Sci U S A. 2007. PMID: 18093961 Free PMC article. - Chronotopographical distribution patterns of cell death and of lectin-positive macrophages/microglial cells during the visual system ontogeny of the small-spotted catshark Scyliorhinus canicula.
Bejarano-Escobar R, Blasco M, Durán AC, Martín-Partido G, Francisco-Morcillo J. Bejarano-Escobar R, et al. J Anat. 2013 Aug;223(2):171-84. doi: 10.1111/joa.12071. Epub 2013 Jun 13. J Anat. 2013. PMID: 23758763 Free PMC article. - Activation of BMP-Smad1/5/8 signaling promotes survival of retinal ganglion cells after damage in vivo.
Ueki Y, Reh TA. Ueki Y, et al. PLoS One. 2012;7(6):e38690. doi: 10.1371/journal.pone.0038690. Epub 2012 Jun 6. PLoS One. 2012. PMID: 22701694 Free PMC article.
References
- Augsburger A, Schuchardt A, Hoskins S, Dodd J, Butler S. BMPs as mediators of roof plate repulsion of commissural neurons. Neuron. 1999;24:127–141. - PubMed
- Barlow AJ, Francis-West PH. Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development. 1997;124:391–398. - PubMed
- Bellusci S, Henderson R, Winnier G, Oikawa T, Hogan BL. Evidence from normal expression and targeted misexpression that bone morphogenetic protein (Bmp-4) plays a role in mouse embryonic lung morphogenesis. Development. 1996;122:1693–1702. - PubMed
- Bovolenta P, Mallamaci A, Puelles L, Boncinelli E. Expression pattern of cSix3, a member of the Six/sine oculis family of transcription factors. Mech Dev. 1998;70:201–203. - PubMed
- Chinnaiyan AM, Dixit VM. The cell-death machine. Curr Biol. 1996;6:555–562. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical