Non-associative learning and serotonin induce similar bi-directional changes in excitability of a neuron critical for learning in the medicinal leech - PubMed (original) (raw)
Non-associative learning and serotonin induce similar bi-directional changes in excitability of a neuron critical for learning in the medicinal leech
B D Burrell et al. J Neurosci. 2001.
Abstract
In studies of the cellular basis of learning, much attention has focused on plasticity in synaptic transmission in terms of transmitter release and the number or responsiveness of neurotransmitter receptors. However, changes in postsynaptic excitability independent of receptors may also play an important role. Changes in excitability of a single interneuron in the leech, the S-cell, were measured during non-associative learning of the whole-body shortening reflex. This interneuron was chosen because it is known to be necessary for sensitization and full dishabituation of the shortening response. During sensitization, S-cell excitability increased, and this enhancement corresponded to facilitation of the shortening reflex and increased S-cell activity during the elicited response. During habituation training, there was a decrement in both the shortening reflex and the elicited S-cell activity, along with decreased S-cell excitability. Conversely, dishabituation facilitated both the shortening response and S-cell activity during shortening, with an accompanying increase in S-cell excitability. Bath application of 1-10 micrometer serotonin (5HT), a modulatory neurotransmitter that is critical for sensitization, for full dishabituation, and for associative learning, increased S-cell excitability. S-cell excitability also increased after stimulation of the serotonergic Retzius cells. However, focal application of serotonin onto the S-cell soma hyperpolarized the interneuron, and bath application of a lower dose of serotonin (0.1 micrometer) decreased excitability. The observed changes in postsynaptic excitability appear to contribute to non-associative learning, and modulatory neurotransmitters, such as serotonin, evidently help regulate excitability. Such changes in S-cell excitability may also be relevant for more complex, associative forms of learning.
Figures
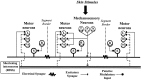
Fig. 1.
Neural circuit of the leech whole-body shortening reflex. Mechanosensory input to the skin is transduced by touch (T), pressure (P), and nociceptive (N) cells, which excite the longitudinal (L), dorsal exciter (DE), and ventral exciter (VE) motor neurons that induce contraction of the longitudinal muscles. The signal to shorten is carried to motor neurons throughout the body by the S-cell and a parallel interneuron known as the R3Sh cell located in the leech head ganglion and, unlike the S-cell, is bilaterally paired (Esch and Kristan, 1999). The sensory input pathways to R3Sh and the excitatory pathways from this interneuron to the motor neurons (dashed lines) are hypothetical. Although the S-cell is active during shortening and has excitatory input onto the L motor neuron (Gardner-Medwin et al., 1973; Magni and Pelligrino, 1978), the S-cell is not necessary for whole-body shortening, but instead is critical for plasticity of this behavior (see introductory remarks). The Retzius (R) cells contain 5HT. Connections marked by_asterisk_ are polysynaptic.
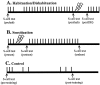
Fig. 2.
Training protocol for non-associative learning experiments. Each vertical line represents a single stimulus–response trial in which a mechanosensory stimulus (a single electroshock) was used to elicit a whole-body shortening response. ITIs are 2 min unless noted otherwise. Lightning bolt symbols_represent noxious stimuli (trains of electroshock pulses) used to sensitize or dishabituate a preparation. For all training conditions, the S-cell excitability and input resistance were measured (prehab, presen, and_pre-training S-cell test) followed by three stimulus–response trials to establish a baseline shortening response for that preparation. A, Preparations were habituated by repetitive mechanosensory stimuli and dishabituated by noxious stimuli. Additional S-cell measurements were made after habituation training (posthab) and after dishabituation (postDH). B, Preparations were sensitized by noxious stimuli. Additional S-cell measurements were made after delivery of the noxious stimuli (postsen) and after repetitive stimulation (endsen). C, After the baseline test, control preparations were stimulated at 4 min ITIs at time points that corresponded to the beginning and end of the habituation training period. S-cell measurements were made before baseline stimulation (pre-training) and at the end of the pseudo-training period (post-training).
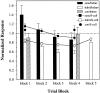
Fig. 3.
Effects of sensitization, habituation, and dishabituation on whole-body shortening (bar plots) and S-cell activity (line/point plots) during shortening. Data are presented as the mean ± SE of the normalized response at each trial block. Each trial block represents the average of five stimulus–response trials and was normalized to an initial response measure made before training. The broken horizontal line represents the initial baseline level for each response measure.
Fig. 4.
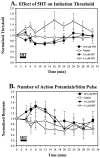
Examples of sensitization- and habituation-induced changes in S-cell excitability. In each set of traces, the top traces are membrane potential measurements during elicited activity, and the bottom traces are the current pulses used. Sensitization decreases (A) the S-cell initiation threshold (measured as the amount of current necessary to produce a single action potential) and increases (B) the number of action potentials produced by a long current pulse. Habituation increases (C) the S-cell initiation threshold and decreases (D) the number of action potentials elicited by the long stimulus pulse.
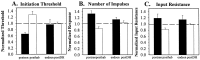
Fig. 5.
Effects of non-associative learning on S-cell response properties. All post-training S-cell measurements taken from sensitization (filled bars) and habituation/dishabituation (open bars) preparations have been normalized to pretraining values and are presented as the mean ± SE. The timing of each measurement taken during a given training protocol is presented in Figure 2. Response properties measured were the initiation threshold (A), the number of action potentials fired during a 200 msec current pulse (B), and the input resistance at the soma (C).
Fig. 6.
5HT concentration series. Effects of saline (○) and with 0.1 μ
m
(▵), 1.0 μ
m
(●), and 10 μ
m
(▴) 5HT on S-cell excitability as measured by initiation threshold (level of 25 msec current pulse) (A) and the number of action potentials elicited by a 250 msec current pulse (B). Values were normalized to those recorded initially for each S-cell.
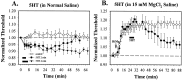
Fig. 7.
Metal microelectrode experiments.A, Effects of 2 min (▴) and 4 min (●) bath application of 1.0 μ
m
5HT on S-cell initiation threshold. S-cells in the control group (○) were constantly perfused with normal saline. B, Effects of 1.0 μ
m
5HT (4 min application) on S-cell excitability in the presence of 15 m
m
MgCl2 saline. S-cell initiation threshold from both the treatment group (●) and the control group (○) was initially tested in normal saline. The normal saline was then replaced with 15 m
m
MgCl2 saline (arrow), and 5HT dissolved in 15 m
m
MgCl2 saline was bath-applied 18 min later. Measurements of initiation threshold were normalized to the initial levels for each experiment.
Fig. 8.
Effects of Retzius cell stimulation on S-cell excitability. S-cells were tested before (0 min) and 2, 5, and 10 min after Retzius cell stimulation at 3–4 Hz (●) and 0.3 Hz (○). Changes in S-cell initiation threshold (A), number of action potentials elicited by a 200 msec current pulse (B), and input resistance (C) were measured. All S-cell measurements were normalized to values taken before Retzius cell stimulation.
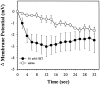
Fig. 9.
Effects of 5HT during direct soma application. Changes in S-cell resting potential immediately after direct application of 50 m
m
5HT (●) or 15 m
m
MgCl2 saline (○) onto the S-cell soma.
Similar articles
- Serotonin mediates learning-induced potentiation of excitability.
Burrell BD, Sahley CL. Burrell BD, et al. J Neurophysiol. 2005 Dec;94(6):4002-10. doi: 10.1152/jn.00432.2005. Epub 2005 Aug 24. J Neurophysiol. 2005. PMID: 16120666 - The S cell: an interneuron essential for sensitization and full dishabituation of leech shortening.
Sahley CL, Modney BK, Boulis NM, Muller KJ. Sahley CL, et al. J Neurosci. 1994 Nov;14(11 Pt 1):6715-21. doi: 10.1523/JNEUROSCI.14-11-06715.1994. J Neurosci. 1994. PMID: 7965072 Free PMC article. - Learning processes in elementary nervous systems§.
Traina G. Traina G. J Integr Neurosci. 2020 Dec 30;19(4):673-678. doi: 10.31083/j.jin.2020.04.318. J Integr Neurosci. 2020. PMID: 33378841 - [Changes of the neuronal membrane excitability as cellular mechanisms of learning and memory].
Gaĭnutdinov KhL, Andrianov VV, Gaĭnutdinova TKh. Gaĭnutdinov KhL, et al. Usp Fiziol Nauk. 2011 Jan-Mar;42(1):33-52. Usp Fiziol Nauk. 2011. PMID: 21442956 Review. Russian. - What we have learned from the study of learning in the leech.
Sahley CL. Sahley CL. J Neurobiol. 1995 Jul;27(3):434-45. doi: 10.1002/neu.480270314. J Neurobiol. 1995. PMID: 7673899 Review.
Cited by
- Differences in chloride gradients allow for three distinct types of synaptic modulation by endocannabinoids.
Wang Y, Burrell BD. Wang Y, et al. J Neurophysiol. 2016 Aug 1;116(2):619-28. doi: 10.1152/jn.00235.2016. Epub 2016 May 25. J Neurophysiol. 2016. PMID: 27226449 Free PMC article. - 5-HT and GABA modulate intrinsic excitability of type I interneurons in Hermissenda.
Jin NG, Tian LM, Crow T. Jin NG, et al. J Neurophysiol. 2009 Nov;102(5):2825-33. doi: 10.1152/jn.00477.2009. Epub 2009 Aug 26. J Neurophysiol. 2009. PMID: 19710377 Free PMC article. - Properties of cannabinoid-dependent long-term depression in the leech.
Li Q, Burrell BD. Li Q, et al. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010 Nov;196(11):841-51. doi: 10.1007/s00359-010-0566-9. Epub 2010 Aug 28. J Comp Physiol A Neuroethol Sens Neural Behav Physiol. 2010. PMID: 20803022 - Initial Variability and Time-Dependent Changes of Neuronal Response Features Are Cell-Type-Specific.
Scherer JS, Riedesel OE, Arkhypchuk I, Meiser S, Kretzberg J. Scherer JS, et al. Front Cell Neurosci. 2022 Apr 27;16:858221. doi: 10.3389/fncel.2022.858221. eCollection 2022. Front Cell Neurosci. 2022. PMID: 35573827 Free PMC article. - Activity-dependent long-term potentiation of intrinsic excitability in hippocampal CA1 pyramidal neurons.
Xu J, Kang N, Jiang L, Nedergaard M, Kang J. Xu J, et al. J Neurosci. 2005 Feb 16;25(7):1750-60. doi: 10.1523/JNEUROSCI.4217-04.2005. J Neurosci. 2005. PMID: 15716411 Free PMC article.
References
- Angstaadt JD, Friesen WO. Modulation of swimming behavior in the medicinal leech: I. Effects of serotonin on the electrical properties of swim-gating cell 204. J Comp Physiol [A] 1993;172:223–234. - PubMed
- Araneda R, Andrade R. 5-Hydroxytrypatamine2 and 5-hydroxytrypatamine1A receptors mediate opposing responses on membrane excitability in rat association cortex. Neuroscience. 1991;40:399–412. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources