Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex - PubMed (original) (raw)
Amygdala regulation of nucleus accumbens dopamine output is governed by the prefrontal cortex
M E Jackson et al. J Neurosci. 2001.
Abstract
A dynamic interaction between the prefrontal cortex (PFC), amygdala, and nucleus accumbens (NAc) may be fundamental to regulation of goal-directed behavior by affective and cognitive processes. This study demonstrates that a mechanism for this triadic relationship is an inhibitory control by prefrontal cortex on accumbal dopamine release during amygdala activation. In freely moving rats, microstimulation of basolateral amygdala at intensities that produced mild behavioral activation produced an expected rapid increase in glutamate efflux in the prefrontal cortex and the nucleus accumbens shell region of the ventral striatum. However, during the stimulation, dopamine release increased only in the prefrontal cortex, not in the nucleus accumbens. An increase in accumbal dopamine release was observed during the stimulation if glutamate activation in the prefrontal cortex was inhibited at either presynaptic or postsynaptic levels. Some behaviors expressed during the stimulation were intensified in animals in which prefrontal cortex glutamate activation was blocked. In addition, these animals continued to express stimulus-induced behaviors after the termination of stimulation, whereas normal poststimulus behaviors such as ambulation and grooming were not displayed as frequently. Considering that dopamine neurotransmission in the nucleus accumbens is thought to play an integral role in goal-directed motor behavior, these findings suggest that the prefrontal cortex influences the behavioral impact of amygdala activation via a concomitant active suppression of accumbal dopamine release. Absence of this cortical influence appears to result in an aberrant pattern of behavioral expression in response to amygdala activation, including behavioral perseveration after stimulus termination.
Figures
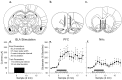
Fig. 1.
Placements of electrode and microdialysis probes, depiction of stimulus parameters, and glutamate efflux during BLA stimulation. Locations of electrode tips (●) and _lines_representing the active surface of microdialysis probes for all animals used in the study are illustrated. Electrode placement was ipsilateral to microdialysis probes in all experiments, but in different rats, placements were made randomly in either the left or right hemisphere.a, Electrode placements were confined to the basolateral amygdala complex. b, PFC probe placements were primarily in infralimbic/prelimbic regions of the medial PFC. c, NAc probe placements were along the border of the shell and medial core. d, BLA stimulation consisted of brief bursts of 50 μA current pulses over a period of 10 min. e, BLA stimulation significantly increased glutamate efflux in the PFC (F = 4.53; p < 0.01;n = 15). f, BLA stimulation also increased glutamate efflux in the NAc (F = 1.69;p < 0.05; n = 10).
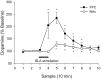
Fig. 2.
Dopamine release during BLA stimulation. BLA stimulation increased extracellular levels of dopamine in both the PFC (F = 6.09; p < 0.01;n = 10) and the NAc (F = 4.65;p < 0.01; n = 10). However,post hoc analysis revealed that NAc dopamine levels did not increase during the period of BLA stimulation when compared with baseline. Asterisks indicate significant differences from baseline (p < 0.05).
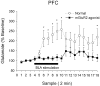
Fig. 3.
Effect of intra-PFC perfusion of mGluR2/3 agonist LY354740 on glutamate efflux in the PFC during BLA stimulation. Reverse dialysis of LY354740 (1 μ
m
) reduced the glutamate efflux evoked by BLA stimulation (solid line;n = 7) as compared with control animals (dotted line; n = 15) (also depicted in Fig. 1). Two-way repeated-measures ANOVA revealed a significant difference between the groups (F = 12.04;p < 0.01). Asterisks indicate individual samples that were significantly different between groups (p < 0.05). [Of note, we were able to detect LY354740 levels in our HPLC system that were used to detect glutamate, and therefore we were able to confirm that LY354740 did not diffuse from the PFC to the NAc because, during the time that the compound was perfused into the PFC, it was not detected in the dialysate obtained from the probe implanted in the NAc (data not shown).]
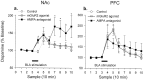
Fig. 4.
Effects of local perfusion into PFC of mGluR2/3 agonist LY354740 (1 μ
m
) and AMPA antagonist LY293558 (100 μ
m
) on dopamine efflux in the NAc and PFC during BLA stimulation. Drug perfusion began at least 1 hr before BLA stimulation.a, BLA stimulation increased NAc dopamine efflux with either the mGluR2/3 agonist in the PFC (solid line, ■) (F = 3.72; p < 0.01;n = 9) or the AMPA antagonist in the PFC (solid line, ▪) (F = 4.26;p < 0.01; n = 6). This increase was in contrast to the lack of immediate dopamine increase during BLA stimulation in control rats (dotted line) (also depicted in Fig. 2.). Asterisks indicate significant differences between the control and the other two groups (p < 0.05). b, In the PFC, dopamine increase was evoked by BLA stimulation in the presence of either the mGluR2/3 agonist (solid line, ■) (F = 11.9; p < 0.01;n = 8) or the AMPA antagonist (solid line, ▪) (F = 2.22; p< 0.05; n = 8). There were no significant differences between these groups and the control group.
Similar articles
- Glutamate receptor-dependent modulation of dopamine efflux in the nucleus accumbens by basolateral, but not central, nucleus of the amygdala in rats.
Howland JG, Taepavarapruk P, Phillips AG. Howland JG, et al. J Neurosci. 2002 Feb 1;22(3):1137-45. doi: 10.1523/JNEUROSCI.22-03-01137.2002. J Neurosci. 2002. PMID: 11826142 Free PMC article. - Stimulus-specific plasticity of prefrontal cortex dopamine neurotransmission.
Jackson ME, Moghaddam B. Jackson ME, et al. J Neurochem. 2004 Mar;88(6):1327-34. doi: 10.1046/j.1471-4159.2003.02205.x. J Neurochem. 2004. PMID: 15009632 - Glutamate-dopamine in vivo interaction in the prefrontal cortex modulates the release of dopamine and acetylcholine in the nucleus accumbens of the awake rat.
Del Arco A, Mora F. Del Arco A, et al. J Neural Transm (Vienna). 2005 Jan;112(1):97-109. doi: 10.1007/s00702-004-0172-5. Epub 2004 Jun 18. J Neural Transm (Vienna). 2005. PMID: 15599608 Review. - Prefrontal cortex-nucleus accumbens interaction: in vivo modulation by dopamine and glutamate in the prefrontal cortex.
Del Arco A, Mora F. Del Arco A, et al. Pharmacol Biochem Behav. 2008 Aug;90(2):226-35. doi: 10.1016/j.pbb.2008.04.011. Epub 2008 Apr 23. Pharmacol Biochem Behav. 2008. PMID: 18508116 Review.
Cited by
- Whole-exome sequencing identifies protein-coding variants associated with brain iron in 29,828 individuals.
Gong W, Fu Y, Wu BS, Du J, Yang L, Zhang YR, Chen SD, Kang J, Mao Y, Dong Q, Tan L, Feng J, Cheng W, Yu JT. Gong W, et al. Nat Commun. 2024 Jul 2;15(1):5540. doi: 10.1038/s41467-024-49702-2. Nat Commun. 2024. PMID: 38956042 Free PMC article. - Incentive sensitization by previous amphetamine exposure: increased cue-triggered "wanting" for sucrose reward.
Wyvell CL, Berridge KC. Wyvell CL, et al. J Neurosci. 2001 Oct 1;21(19):7831-40. doi: 10.1523/JNEUROSCI.21-19-07831.2001. J Neurosci. 2001. PMID: 11567074 Free PMC article. - Triadic model of the neurobiology of motivated behavior in adolescence.
Ernst M, Pine DS, Hardin M. Ernst M, et al. Psychol Med. 2006 Mar;36(3):299-312. doi: 10.1017/S0033291705005891. Psychol Med. 2006. PMID: 16472412 Free PMC article. Review. - Methylphenidate-induced activation of the anterior cingulate but not the striatum: a [15O]H2O PET study in healthy volunteers.
Udo de Haes JI, Maguire RP, Jager PL, Paans AM, den Boer JA. Udo de Haes JI, et al. Hum Brain Mapp. 2007 Jul;28(7):625-35. doi: 10.1002/hbm.20293. Hum Brain Mapp. 2007. PMID: 17080442 Free PMC article. Clinical Trial. - Amphetamine exposure selectively enhances hippocampus-dependent spatial learning and attenuates amygdala-dependent cue learning.
Ito R, Canseliet M. Ito R, et al. Neuropsychopharmacology. 2010 Jun;35(7):1440-52. doi: 10.1038/npp.2010.14. Epub 2010 Mar 3. Neuropsychopharmacology. 2010. PMID: 20200510 Free PMC article.
References
- Bagley J, Moghaddam B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: effects of pretreatment with saline or diazepam. Neuroscience. 1997;77:65–73. - PubMed
- Battaglia G, Monn JA, Schoepp DD. In vivo inhibition of veratridine-evoked release of striatal excitatory amino acids by the group II metabotropic glutamate receptor agonist LY354740 in rats. Neurosci Lett. 1997;229:161–164. - PubMed
- Cador M, Robbins T, Everitt B. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- T32 NS007224/NS/NINDS NIH HHS/United States
- T32NS07224/NS/NINDS NIH HHS/United States
- R01 MH048404/MH/NIMH NIH HHS/United States
- MH44866/MH/NIMH NIH HHS/United States
- MH48404/MH/NIMH NIH HHS/United States
- K02MH01616/MH/NIMH NIH HHS/United States
- R37 MH048404/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous