Temperature-sensitive transformation by an Abelson virus mutant encoding an altered SH2 domain - PubMed (original) (raw)
Temperature-sensitive transformation by an Abelson virus mutant encoding an altered SH2 domain
C A Mainville et al. J Virol. 2001 Feb.
Abstract
Abelson murine leukemia virus (Ab-MLV) encodes the v-Abl protein tyrosine kinase and induces transformation of immortalized fibroblast lines and pre-B cells. Temperature-sensitive mutations affecting the kinase domain of the protein have demonstrated that the kinase activity is absolutely required for transformation. Despite this requirement, mutations affecting other regions of v-Abl modulate transformation activity. The SH2 domain and the highly conserved FLVRES motif within it form a phosphotyrosine-binding pocket that is required for interactions between the kinase and cellular substrates. To understand the impact of SH2 alterations on Ab-MLV-mediated transformation, we studied the Ab-MLV mutant P120/R273K. This mutant encodes a v-Abl protein in which the beta B5 arginine at the base of the phosphotyrosine-binding pocket has been replaced by a lysine. Unexpectedly, infection of NIH 3T3 or pre-B cells with P120/R273K revealed a temperature-dependent transformation phenotype. At 34 degrees C, P120/R273K transformed about 10-fold fewer cells than wild-type virus of equivalent titer; at 39.5 degrees C, 300-fold fewer NIH 3T3 cells were transformed and pre-B cells were refractory to transformation. Temperature-dependent transformation was accompanied by decreased phosphorylation of Shc, a protein that interacts with the v-Abl SH2 and links the protein to Ras, and decreased induction of c-Myc expression. These data suggest that alteration of the FLVRES pocket affects the ability of v-Abl to interact with at least some of its substrates in a temperature-dependent fashion and identify a novel type of temperature-sensitive Abelson virus.
Figures
FIG. 1
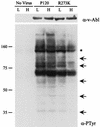
Cells expressing P120/R273K contain low levels of phosphotyrosine at high temperature. NIH 3T3 cells were infected with Ab-MLV P120 or P120/R273K or were mock infected at 34°C (L); some samples were then shifted to 39.5°C (H), and all were lysed 48 h later. Equivalent amounts of total cellular protein were analyzed by using Western blotting with antiphosphotyrosine antibodies or the anti-Gag/v-Abl monoclonal antibody H548 (10). Molecular weight standards (in thousands) are indicated on the left, and arrows highlight bands which display temperature-dependent differences in phosphotyrosine. The asterisk indicates the v-Abl protein.
FIG. 2
Shc and p62Dok bind poorly to an SH2 domain with the R273K substitution. (A) Lysates of uninfected NIH 3T3 cells or cells transformed with Ab-MLV P120 were incubated with GST alone, GST-SH2, or GST-R273K SH2. The proteins were recovered and analyzed by using Western blotting with an anti-Shc antibody. (B) Lysates of NIH 3T3 cells transformed by Ab-MLV P120 were incubated with the indicated GST-SH2 domain fusion proteins or GST protein as a control. The recovered proteins were analyzed by using Western blotting with an anti-p62Dok antibody. All samples contained equivalent amounts of GST proteins (data not shown).
FIG. 3
Tyrosine phosphorylation of Shc and Shc-Grb2 complexes are reduced in cells expressing P120/R273K at 39.5°C. NIH 3T3 cells were infected with Ab-MLV P120 or P120/R273K or were mock infected and incubated at 34°C (L) or 39.5°C (H). (A) The cells were lysed 48 h later, and a portion of the lysate was immunoprecipitated with anti-Shc antibody or rabbit gamma globulin as a control and analyzed by using Western blotting and antiphosphotyrosine, anti-Grb2, and anti-Shc antibodies. Lanes labeled “Shc” contain samples immunoprecipitated with anti-Shc antibody; lanes labeled “C” contain samples immunoprecipitated with rabbit gamma globulin. The antibodies used to probe the blots are listed to the right. (B) The remaining lysate was analyzed by using Western blotting with the anti-Gag/v-Abl monoclonal antibody H548 (10).
FIG. 4
Tyrosine phosphorylation of p62Dok is not temperature dependent. NIH 3T3 cells were infected with Ab-MLV P120 or P120/R273K or were mock infected and incubated at 34°C (L) or 39.5°C (H), and lysates were prepared 48 h later. (A) A portion of the lysate was immunoprecipitated with anti-p62Dok or the UPC-10 control antibody (2) and analyzed by using antiphosphotyrosine and anti-p62Dok antibodies. Lanes labeled “p62” contain samples immunoprecipitated with anti-p62Dok antibody; lanes labeled “C” contain samples immunoprecipitated with UPC-10 control antibody. The antibodies used to probe the blots are listed to the right. (B) The remaining lysate was analyzed by using Western blotting with the anti-Gag/v-Abl monoclonal antibody H548 (10).
FIG. 5
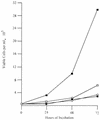
Pre-B cells transformed by P120/R273K grow poorly at 39.5°C. Bone marrow cells were transformed by Ab-MLV P120 (squares) or P120/R273K (circles) at 34°C, and primary transformants were expanded and established. Cells were counted and seeded in 35-mm dishes at a density of 105 cells per ml and incubated at 34°C (open symbols) or 39.5°C (filled symbols). Duplicate cultures were counted, and viable cells were enumerated using phase microscopy daily for 3 days. The experiments shown are representative of at least three experiments in which two or more cell lines transformed with each virus were analyzed.
FIG. 6
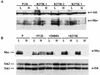
Expression of c-Myc is decreased in cells expressing P120/R273K at 39.5°C. Pre-B-cell lines transformed at 34°C by Ab-MLV P120 or P120/R273K (A) or 7C411 parental cells (P) expressing P120, P120/D484N, or P120/R273K (B) were maintained at 34°C or shifted to 39.5°C for 48 h, and lysates were prepared. The samples were analyzed via Western blotting using anti-c-Myc. The anti-Gag/v-Abl monoclonal antibody H548 (10) was used as a loading control in the top panel; anti-Erk antibody was used as a loading control in the bottom panel. In panel A, three representative pre-B-cell lines transformed with P120/R273K are shown; in panel B, two representative clones expressing P120/R273K are shown.
Similar articles
- Absence of p53 complements defects in Abelson murine leukemia virus signaling.
Unnikrishnan I, Rosenberg N. Unnikrishnan I, et al. J Virol. 2003 Jun;77(11):6208-15. doi: 10.1128/jvi.77.11.6208-6215.2003. J Virol. 2003. PMID: 12743277 Free PMC article. - The carboxyl terminus of v-Abl protein can augment SH2 domain function.
Warren D, Heilpern AJ, Berg K, Rosenberg N. Warren D, et al. J Virol. 2000 May;74(10):4495-504. doi: 10.1128/jvi.74.10.4495-4504.2000. J Virol. 2000. PMID: 10775585 Free PMC article. - Active Akt and functional p53 modulate apoptosis in Abelson virus-transformed pre-B cells.
Gong L, Unnikrishnan I, Raghavan A, Parmar K, Rosenberg N. Gong L, et al. J Virol. 2004 Feb;78(4):1636-44. doi: 10.1128/jvi.78.4.1636-1644.2004. J Virol. 2004. PMID: 14747529 Free PMC article. - Inhibition of Abelson oncogene function by erbstatin analogues.
Kawada M, Tawara J, Tsuji T, Honma Y, Hozumi M, Wang JY, Umezawa K. Kawada M, et al. Drugs Exp Clin Res. 1993;19(6):235-41. Drugs Exp Clin Res. 1993. PMID: 8013266 Review. - Transforming pathways activated by the v-Abl tyrosine kinase.
Shore SK, Tantravahi RV, Reddy EP. Shore SK, et al. Oncogene. 2002 Dec 9;21(56):8568-76. doi: 10.1038/sj.onc.1206084. Oncogene. 2002. PMID: 12476303 Review.
Cited by
- p16(Ink4a) interferes with Abelson virus transformation by enhancing apoptosis.
Sachs Z, Sharpless NE, DePinho RA, Rosenberg N. Sachs Z, et al. J Virol. 2004 Apr;78(7):3304-11. doi: 10.1128/jvi.78.7.3304-3311.2004. J Virol. 2004. PMID: 15016851 Free PMC article. - Absence of p53 complements defects in Abelson murine leukemia virus signaling.
Unnikrishnan I, Rosenberg N. Unnikrishnan I, et al. J Virol. 2003 Jun;77(11):6208-15. doi: 10.1128/jvi.77.11.6208-6215.2003. J Virol. 2003. PMID: 12743277 Free PMC article. - Gag influences transformation by Abelson murine leukemia virus and suppresses nuclear localization of the v-Abl protein.
Yi CR, Rosenberg N. Yi CR, et al. J Virol. 2007 Sep;81(17):9461-8. doi: 10.1128/JVI.00735-07. Epub 2007 Jun 27. J Virol. 2007. PMID: 17596313 Free PMC article. - SH2-containing inositol 5'-phosphatase inhibits transformation of Abelson murine leukemia virus.
Fessler SP, Rosenberg N, Baughn LB. Fessler SP, et al. J Virol. 2011 Sep;85(17):9239-42. doi: 10.1128/JVI.05115-11. Epub 2011 Jun 22. J Virol. 2011. PMID: 21697469 Free PMC article. - Mutations affecting the MA portion of the v-Abl protein reveal a conserved role of Gag in Abelson murine leukemia virus (MLV) and Moloney MLV.
Yi CR, Rosenberg N. Yi CR, et al. J Virol. 2008 Jun;82(11):5307-15. doi: 10.1128/JVI.00089-08. Epub 2008 Mar 26. J Virol. 2008. PMID: 18367522 Free PMC article.
References
- Afar D E H, Goga A, McLaughlin J, Witte O N, Sawyers C L. Differential complementation of Bcr-Abl point mutants with c-Myc. Science. 1994;264:424–426. - PubMed
- Auffray C, Sikorav J L, Rougeon F. Correlation between D region structure and antigen-binding specificity: evidences for the comparison of closely related immunoglobulin VH sequences. Ann Immunol. 1981;132D:77–78. - PubMed
- Bradshaw J M, Mitaxov V, Waksman G. Investigation of phosphotyrosine recognition by the SH2 domain of the Src kinase. J Mol Biol. 1999;293:971–985. - PubMed
- Bradshaw J M, Mitaxov V, Waksman G. Mutational investigation of the specificity determining region of the Src SH2 domain. J Mol Biol. 2000;299:521–535. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous