Mice lacking the ERK1 isoform of MAP kinase are unimpaired in emotional learning - PubMed (original) (raw)
Mice lacking the ERK1 isoform of MAP kinase are unimpaired in emotional learning
J C Selcher et al. Learn Mem. 2001 Jan-Feb.
Abstract
The extracellular signal-regulated kinases (ERKs) are members of the mitogen-activated protein kinase (MAPK) superfamily of enzymes and have recently garnered considerable attention in the field of learning and memory. ERK activation has been shown to be required for the induction of long-term potentiation (LTP) in the rat hippocampus and for the formation of associative and spatial memories in both the rat and the mouse. However, the individual roles for the two isoforms of ERK have yet to be deciphered. To investigate the specific contribution of the ERK1 (p44) isoform of MAPK to mammalian learning, we performed a general behavioral and physiological characterization of mice lacking the ERK1 gene. The ERK1-null animals demonstrated significantly higher levels of activity in the open field test. However, we observed no other discernible deficits in the ERK1 knockout mice in our behavioral testing. Specifically, no differences were observed in the acquisition or retention (24 h and 2 wk after training) of either contextual or cue fear conditioning between the ERK1(-/-) and their wild-type littermate controls. In addition, no learning phenotype was observed in the passive avoidance test. When hippocampal slices were analyzed, we found no deficits in baseline synaptic transmission or in tetanus-induced LTP in hippocampal area CA1. We found no apparent compensatory changes in the expression of ERK2 (p42 MAPK). We conclude that hippocampus- and amygdala-dependent emotional learning does not depend critically on the activity of ERK1.
Figures
Figure 1
ERK1-null animals are developmentally normal. (A) Sagittal sections through the brains of adult wild-type and ERK1−/− mice at 25x magnification. (B) Representative Western blots showing ERK protein expression in tissue from various regions of the CNS from ERK1 knockout and wild-type mice. (C) Western blots showing phospho-ERK (top) and total ERK (bottom) protein expression in 400–μm-thick hippocampal slices prepared from ERK1-null mice and littermate wildtypes. The first two lanes show tissue from control slices. Hippocampal tissue in the middle two lanes received a 10 min exposure to 50 μM forskolin (+FSK), while tissue in the last two lanes were exposed to 50 μM forskolin following a 1 h pre-incubation with 20 μM U0126 (+FSK/U0126).
Figure 2
ERK1 knockout mice show increased horizontal activity in the open field. (A,B) Horizontal activity measured by the number of beam breaks and total distance respectively for ERK1−/− (open box; n = 14) versus wild-type (filled circle; n = 12) mice. (C) Vertical activity was not different between ERK1−/− and wild-type mice. (D) No difference in center distance-to-total distance ratio for ERK1−/− and wild-type controls. Data are represented as the mean (± s.e.m.).
Figure 3
Mice deficient in ERK1 show no impairment on the rotarod task. There was no significant difference between the amount of time ERK1−/− (open box; n = 10) and wild-type (filled circle; n = 8) mice spent balanced on the accelerating rotarod. Performance in both sets of mice improved significantly over the course of eight trials.
Figure 4
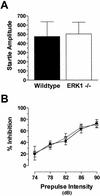
No difference in startle amplitude or prepulse inhibition between ERK1−/− and wild-type mice. (A) Acoustic startle amplitude to the 120-dB stimulus for wild-type and ERK1 knockout mice. (B) Inhibition of the startle response by prepulses of varying intensities for ERK1−/− (open box; n = 14) and wild-type (filled circle; n = 12) mice.
Figure 5
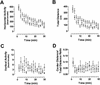
ERK1 is not required for normal fear conditioning. (A) Freezing responses during the training phase are shown. Within a novel environment, an acoustic stimulus (solid bar) was paired twice with a footshock (arrow) during training. Baseline behavior (before presentation of the tone) and shock response (after the footshock) were similar for both ERK1−/− ( open box; n = 10) and wild-type (filled circle; n = 8) groups. (B) Compared to controls, ERK1-deficient mice demonstrated normal freezing to the context 24 h after receiving two pairings of tone and shock. (C) Freezing in response to re-presentation of the auditory CS (solid bar) in a different context 24 h after training also revealed unimpaired cue learning in ERK1 knockout mice. (D) Contextual fear conditioning was still intact when tested 14 d after training in both ERK1−/− and wild-type mice. (E) Re-presentation of the auditory CS (solid bar) 14 d after fear conditioning yielded freezing levels in ERK1−/− mice similar to wild-type controls. These results suggest unimpaired long-term retention of fear learning.
Figure 6
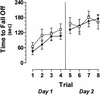
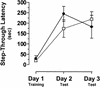
ERK1-deficient mice are unimpaired in passive avoidance learning. Time to enter the dark half of the chamber, or step-through latency, was similar on both testing days for ERK1−/− mice ( open box; n = 10) and littermate wildtype controls (filled circle; n = 7).
Figure 7
Normal synaptic transmission and synaptic plasticity in ERK1 knockouts. (A) Baseline synaptic transmission shown with input-output curves for ERK1−/− (open box; n = 9 slices) and littermate wildtype controls (filled circle; n = 10). (B) Paired-pulse facilitation in hippocampal slices from ERK1-deficient and wild-type mice. (C) LTP induced with a pair of 100-Hz tetani in area CA1 is similar for ERK1−/− and wild-type hippocampal slices. Inset, representative traces from control and mutant slices before (gray) and after (black) tetanic stimulation. Scale bars are 0.2 mV by 4 msec. The arrow indicates the delivery of two trains of 100-Hz, 1-sec stimulation.
Similar articles
- A role for ERK MAP kinase in physiologic temporal integration in hippocampal area CA1.
Selcher JC, Weeber EJ, Christian J, Nekrasova T, Landreth GE, Sweatt JD. Selcher JC, et al. Learn Mem. 2003 Jan-Feb;10(1):26-39. doi: 10.1101/lm.51103. Learn Mem. 2003. PMID: 12551961 Free PMC article. - Neuronal MEK is important for normal fear conditioning in mice.
Shalin SC, Zirrgiebel U, Honsa KJ, Julien JP, Miller FD, Kaplan DR, Sweatt JD. Shalin SC, et al. J Neurosci Res. 2004 Mar 15;75(6):760-70. doi: 10.1002/jnr.20052. J Neurosci Res. 2004. PMID: 14994337 - Knockout of ERK1 MAP kinase enhances synaptic plasticity in the striatum and facilitates striatal-mediated learning and memory.
Mazzucchelli C, Vantaggiato C, Ciamei A, Fasano S, Pakhotin P, Krezel W, Welzl H, Wolfer DP, Pagès G, Valverde O, Marowsky A, Porrazzo A, Orban PC, Maldonado R, Ehrengruber MU, Cestari V, Lipp HP, Chapman PF, Pouysségur J, Brambilla R. Mazzucchelli C, et al. Neuron. 2002 May 30;34(5):807-20. doi: 10.1016/s0896-6273(02)00716-x. Neuron. 2002. PMID: 12062026 - The integrated role of ACh, ERK and mTOR in the mechanisms of hippocampal inhibitory avoidance memory.
Giovannini MG, Lana D, Pepeu G. Giovannini MG, et al. Neurobiol Learn Mem. 2015 Mar;119:18-33. doi: 10.1016/j.nlm.2014.12.014. Epub 2015 Jan 13. Neurobiol Learn Mem. 2015. PMID: 25595880 Review. - The use of null mutant mice to study complex learning and memory processes.
Wehner JM, Bowers BJ, Paylor R. Wehner JM, et al. Behav Genet. 1996 May;26(3):301-12. doi: 10.1007/BF02359386. Behav Genet. 1996. PMID: 8754253 Review.
Cited by
- Redundancy in the World of MAP Kinases: All for One.
Saba-El-Leil MK, Frémin C, Meloche S. Saba-El-Leil MK, et al. Front Cell Dev Biol. 2016 Jun 27;4:67. doi: 10.3389/fcell.2016.00067. eCollection 2016. Front Cell Dev Biol. 2016. PMID: 27446918 Free PMC article. Review. - Emerging role of MAP kinase pathways as therapeutic targets in COPD.
Mercer BA, D'Armiento JM. Mercer BA, et al. Int J Chron Obstruct Pulmon Dis. 2006;1(2):137-50. doi: 10.2147/copd.2006.1.2.137. Int J Chron Obstruct Pulmon Dis. 2006. PMID: 18046891 Free PMC article. Review. - Extracellular signal-regulated kinase 2 mRNA expression in the rat brain during aging.
Simonyi A, Murch K, Sun GY. Simonyi A, et al. Neurochem Res. 2003 Sep;28(9):1375-8. doi: 10.1023/a:1024948532633. Neurochem Res. 2003. PMID: 12938860 - Local knockdown of ERK2 in the adult mouse brain via adeno-associated virus-mediated RNA interference.
Di Benedetto B, Wefers B, Wurst W, Kühn R. Di Benedetto B, et al. Mol Biotechnol. 2009 Mar;41(3):263-9. doi: 10.1007/s12033-008-9125-9. Epub 2008 Dec 4. Mol Biotechnol. 2009. PMID: 19052925 Free PMC article. - ERK/MAPK Signaling Is Required for Pathway-Specific Striatal Motor Functions.
Hutton SR, Otis JM, Kim EM, Lamsal Y, Stuber GD, Snider WD. Hutton SR, et al. J Neurosci. 2017 Aug 23;37(34):8102-8115. doi: 10.1523/JNEUROSCI.0473-17.2017. Epub 2017 Jul 21. J Neurosci. 2017. PMID: 28733355 Free PMC article.
References
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. - PubMed
- Abeliovich A, Paylor R, Chen C, Kim JJ, Wehner JM, Tonegawa S. PKCg mutant mice exhibit mild deficits in spatial and contextual learning. Cell. 1993;75:1263–1271. - PubMed
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous