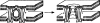Osteoclasts and giant cells: macrophage-macrophage fusion mechanism - PubMed (original) (raw)
Review
Osteoclasts and giant cells: macrophage-macrophage fusion mechanism
A Vignery. Int J Exp Pathol. 2000 Oct.
Abstract
Membrane fusion is a ubiquitous event that occurs in a wide range of biological processes. While intracellular membrane fusion mediating organelle trafficking is well understood, much less is known about cell-cell fusion mediating sperm cell-oocyte, myoblast-myoblast and macrophage-macrophage fusion. In the case of mononuclear phagocytes, their fusion is not only associated with the differentiation of osteoclasts, cells which play a key role in the pathogenesis of osteoporosis, but also of giant cells that are present in chronic inflammatory reactions and in tumours. Despite the biological and pathophysiological importance of intercellular fusion events, the actual molecular mechanism of macrophage fusion is still unclear. One of the main research themes in my laboratory has been to investigate the molecular mechanism of mononuclear phagocyte fusion. Our hypothesis has been that macrophage-macrophage fusion, similar to virus-cell fusion, is mediated by specific cell surface proteins. But, in contrast with myoblasts and sperm cells, macrophage fusion is a rare event that occurs in specific instances. To test our hypothesis, we established an in vitro cell-cell fusion assay as a model system which uses alveolar macrophages. Upon multinucleation, these macrophages acquire the osteoclast phenotype. This indicates that multinucleation of macrophages leads to a specific and novel functional phenotype in macrophages. To identify the components of the fusion machinery, we generated four monoclonal antibodies (mAbs) which block the fusion of alveolar macrophages and purified the unique antigen recognized by these mAbs. This led us to the cloning of MFR (Macrophage Fusion Receptor). MFR was cloned simultaneously as P84/SHPS-1/SIRPalpha/BIT by other laboratories. We subsequently showed that the recombinant extracellular domain of MFR blocks fusion. Most recently, we identified a lower molecular weight form of MFR that is missing two extracellular immunoglobulin (Ig) C domains. Shortly after we cloned MFR, CD47 was reported to be a ligand for P84/SIRPalpha. We have since generated preliminary results which suggest that CD47 interacts with MFR during adhesion/fusion and is a member of the fusion machinery. We also identified CD44 as a plasma membrane protein which, like MFR, is highly expressed at the onset of fusion. The recombinant soluble extracellular domain of CD44 blocks fusion by interacting with a cell-surface binding site. We now propose a model in which both forms of MFR, CD44, and CD47 mediate macrophage adhesion/fusion and therefore the differentiation of osteoclasts and giant cells.
Figures
Figure 1
Subunit organization of the fusion glycoproteins of influenza Sendai, human immunodeficiency, and Rous Sarcoma viruses. Hatched boxes represent fusion peptides, filled black boxes represent transmembrane domains. Redrawn after Hernandez et al. (1996).
Figure 2
A schematic hypothetical model of the postulated fusion site created during influenza virus HA-mediated fusion. Left, the association of several unfolded HA trimers is proposed to dehydrate the intermediate space, thereby forming an intermembrane intermediate. Right, rupture of the intermembrane intermediate perpendicular to the plane of the membranes would create a small pore, or interlamellar attachment site, thereby causing bilayer fusion. Reprinted after Hernandez et al. (1996).
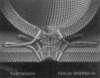
Figure 3
Hypothetical model of the synaptic fusion complex as it joins two membranes. Reprinted after Sutton et al. (1998).
Figure 4
Hypothetical model of the synaptic fusion complex as it joins two membranes. In contrast to viral hairpins (fusion proteins, left), cellular SNAREpins (right) are formed from separate polypeptides that reside in different membranes before fusion. The similarity between SNAREpins (in which pins represent the hydrophobic domain of the polypeptide) and viral hairpins suggests that they all employ a fundamentally similar mechanism to coalesce lipid bilayers. Reprinted after Weber et al. (1998).
Figure 5
Macrophage Fusion Model System. Rat alveolar macrophages plated at high density on day 0, and on day 3, when fusion is 99% complete (c). While the expression of osteoclast markers increases with multinucleation (a), that of MFR and CD44 decreases (b).
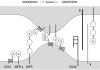
Figure 6
Hypothetical Macrophage Fusion Mechanism. Macrophage–macrophage adhesion is secured by MFR and CD44, interacting either directly or indirectly, via other unknown ligands (X and Y). The stepwise association between the long form of MFR with CD47, followed by the short form of MFR with CD47 could reduce the distance between the cells down to 5–10 nm. That distance may be further reduced if MFR and CD47 bend upon binding. In parallel, it is conceivable that the association between MFRs and CD47 in the same cell secures the mononucleated state of macrophages.
Similar articles
- CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation.
Han X, Sterling H, Chen Y, Saginario C, Brown EJ, Frazier WA, Lindberg FP, Vignery A. Han X, et al. J Biol Chem. 2000 Dec 1;275(48):37984-92. doi: 10.1074/jbc.M002334200. J Biol Chem. 2000. PMID: 10964914 - MFR, a putative receptor mediating the fusion of macrophages.
Saginario C, Sterling H, Beckers C, Kobayashi R, Solimena M, Ullu E, Vignery A. Saginario C, et al. Mol Cell Biol. 1998 Nov;18(11):6213-23. doi: 10.1128/MCB.18.11.6213. Mol Cell Biol. 1998. PMID: 9774638 Free PMC article. - SHPS-1 induces aggregation of Ba/F3 pro-B cells via an interaction with CD47.
Babic I, Schallhorn A, Lindberg FP, Jirik FR. Babic I, et al. J Immunol. 2000 Apr 1;164(7):3652-8. doi: 10.4049/jimmunol.164.7.3652. J Immunol. 2000. PMID: 10725722 - Role of DC-STAMP in cellular fusion of osteoclasts and macrophage giant cells.
Yagi M, Miyamoto T, Toyama Y, Suda T. Yagi M, et al. J Bone Miner Metab. 2006;24(5):355-8. doi: 10.1007/s00774-006-0697-9. J Bone Miner Metab. 2006. PMID: 16937266 Review. - Macrophage fusion: are somatic and cancer cells possible partners?
Vignery A. Vignery A. Trends Cell Biol. 2005 Apr;15(4):188-93. doi: 10.1016/j.tcb.2005.02.008. Trends Cell Biol. 2005. PMID: 15817374 Review.
Cited by
- Enforced adhesion of hematopoietic cells to culture dish induces endomitosis and polyploidy.
Huang X, Dai W, Darzynkiewicz Z. Huang X, et al. Cell Cycle. 2005 Jun;4(6):801-5. doi: 10.4161/cc.4.6.1695. Epub 2005 Jun 15. Cell Cycle. 2005. PMID: 15908784 Free PMC article. - Blastulation of a zygote to a hatched blastocyst without any clear cell division: an observational finding in a time-lapse system after in vitro fertilization.
Sandi-Monroy NL, Musanovic S, Zhu D, Eibner K, Reeka N, Koglin J, Bundschu K, Gagsteiger F. Sandi-Monroy NL, et al. J Assist Reprod Genet. 2020 Mar;37(3):693-697. doi: 10.1007/s10815-020-01704-x. Epub 2020 Feb 6. J Assist Reprod Genet. 2020. PMID: 32026203 Free PMC article. - Fusion of microglia with pyramidal neurons after retroviral infection.
Ackman JB, Siddiqi F, Walikonis RS, LoTurco JJ. Ackman JB, et al. J Neurosci. 2006 Nov 1;26(44):11413-22. doi: 10.1523/JNEUROSCI.3340-06.2006. J Neurosci. 2006. PMID: 17079670 Free PMC article. - Cell-to-cell spread and massive vacuole formation after Cryptococcus neoformans infection of murine macrophages.
Alvarez M, Casadevall A. Alvarez M, et al. BMC Immunol. 2007 Aug 16;8:16. doi: 10.1186/1471-2172-8-16. BMC Immunol. 2007. PMID: 17705844 Free PMC article. - Stimulation of osteoclast formation by inflammatory synovial fluid.
Adamopoulos IE, Danks L, Itonaga I, Locklin RM, Sabokbar A, Ferguson DJ, Athanasou NA. Adamopoulos IE, et al. Virchows Arch. 2006 Jul;449(1):69-77. doi: 10.1007/s00428-006-0200-y. Epub 2006 Apr 21. Virchows Arch. 2006. PMID: 16642388
References
- Alkhatib G, Combadiere C, Broder C, et al. CCCKR5: a RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science. 1996;272:1955–1958. - PubMed
- Baron R. Molecular mechanisms of bone resorption: therapeutic implications. Acta Orthop Scand Supplement. 1995;266:66–70. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous