Surface-localized glycine transporters 1 and 2 function as monomeric proteins in Xenopus oocytes - PubMed (original) (raw)
Surface-localized glycine transporters 1 and 2 function as monomeric proteins in Xenopus oocytes
M Horiuchi et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Na(+)/Cl(-)-dependent neurotransmitter transporters form a superfamily of transmembrane proteins that share 12 membrane-spanning regions. To gain information about the quaternary structure of these transporter proteins, we heterologously expressed the glial glycine transporter GlyT1 and its neuronal homolog GlyT2 in Xenopus oocytes. By using metabolic labeling with [(35)S]methionine or surface labeling with a plasma membrane impermeable reagent followed by affinity purification, we separately analyzed the total cellular pools of newly synthesized GlyTs and its functional plasma membrane-bound fractions. Upon blue native gel electrophoresis, the surface-localized transporter proteins were found to exist exclusively in complex-glycosylated monomeric form, whereas a significant fraction of the intracellular GlyT1 and GlyT2 was core-glycosylated and oligomeric. In contrast, even after treatment with the crosslinker glutaraldehyde, surface GlyTs failed to migrate as oligomeric proteins. These results indicate that plasma membrane-bound GlyT1 and GlyT2 are monomeric proteins. Thus, Na(+)/Cl(-)-dependent neurotransmitter transporters do not require oligomerization for substrate translocation.
Figures
Figure 1
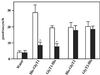
Transport activity of heterologously expressed His6-tagged GlyTs. Oocytes injected with 25 ng of the indicated cRNAs or water were kept at 19°C for 48 h. Groups of four oocytes each were then incubated with 10 μM [3H]glycine in the absence (open column) or presence (closed column) of 1 mM sarcosine for determination of [3H]glycine uptake. The data show the means ± SD from four groups of oocytes for each experimental condition, with uptake values of 28.7 ± 3.4 and 19.3 ± 1.2 pmol/oocyte/h for His-GlyT1 and GlyT1-His, and of 19.7 ± 2.9 and 20.1 ± 3.2 pmol/oocyte/h for His-GlyT2 and GlyT2-His, respectively. These values correspond to 60–129% of the uptake found with the wild-type GlyT1 and GlyT2 proteins under identical conditions. Statistically significant differences between oocytes incubated with and without sarcosine are indicated (*, P < 0.01).
Figure 2
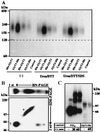
BN/PAGE of metabolically and surface-labeled GlyTs. (Left) Oocytes injected with 25 ng of the indicated cRNAs were labeled metabolically with [35S]methionine or surface-labeled with [125I]sulfo-SHPP, respectively. After solubilization with 1% (wt/vol) digitonin, the His6-tagged GlyT proteins were isolated by Ni2+-NTA chromatography and resolved by 4–13% BN/PAGE. (Right) For direct comparison, the [35S]methionine-labeled and [125I]-labeled GlyT1-His preparations were separated on adjacent lanes of a native 4–13% polyacrylamide gradient gel.
Figure 3
SDS/PAGE of metabolically (Left) and surface-labeled (Right) GlyTs before and after deglycosylation. Affinity-purified GlyT proteins were denatured in reducing SDS sample buffer, incubated for 2 h or 24 h, at 37°C with the enzymes indicated (5 units of endo H, 2.5 units of PNGase F), and analyzed on an 8% polyacrylamide gel. Different deglycosylation intermediates of GlyT1 and its fully deglycosylated form are indicated by 1–3 and 0, respectively. * and †, complex-glycosylated and core-glycosylated GlyT polypeptides.
Figure 4
Surface GlyTs migrate as monomeric proteins. (A) Effect of reduction, urea, and SDS on the mobility of surface-labeled GlyT proteins. After surface labeling with [15I]sulfo-SHPP and affinity purification, the 125I-labeled GlyTs were incubated at room temperature either alone (Left) or with 100 mM DTT and 8 M urea in the absence (Center) or presence (Right) of 0.1% (wt/vol) SDS, respectively, before analysis by 4–10% BN/PAGE. Note that the mobility of the labeled proteins did not change significantly under the different conditions. (B) An aliquot of a detergent extract prepared from an GlyT1-His-injected oocyte after metabolic labeling with [35S]methionine as shown in Fig. 2 was subjected to two-dimensional PAGE. After BN/PAGE on a 4–10% acrylamide gradient gel, the separated proteins were resolved in the second dimension by 8% SDS/PAGE. * and †, the complex-glycosylated and core-glycosylated forms, respectively. (C) Surface-labeled oocytes expressing either the P2X3 receptor or GlyT1-His were incubated with 10 mM glutaraldehyde (GA) for the indicated periods before extraction and affinity purification. The purified proteins were then analyzed on a 4–10% SDS/polyacrylamide gradient gel. Note the formation of P2X3 dimers and trimers, whereas no adducts were seen with GlyT1-His.
Similar articles
- Cloning and expression of a spinal cord- and brain-specific glycine transporter with novel structural features.
Liu QR, López-Corcuera B, Mandiyan S, Nelson H, Nelson N. Liu QR, et al. J Biol Chem. 1993 Oct 25;268(30):22802-8. J Biol Chem. 1993. PMID: 8226790 - Glycine transporters GlyT1 and GlyT2 are differentially modulated by glycogen synthase kinase 3β.
Jiménez E, Núñez E, Ibáñez I, Zafra F, Aragón C, Giménez C. Jiménez E, et al. Neuropharmacology. 2015 Feb;89:245-54. doi: 10.1016/j.neuropharm.2014.09.023. Epub 2014 Oct 6. Neuropharmacology. 2015. PMID: 25301276 - An aspartate residue in the external vestibule of GLYT2 (glycine transporter 2) controls cation access and transport coupling.
Pérez-Siles G, Núñez E, Morreale A, Jiménez E, Leo-Macías A, Pita G, Cherubino F, Sangaletti R, Bossi E, Ortíz AR, Aragón C, López-Corcuera B. Pérez-Siles G, et al. Biochem J. 2012 Mar 1;442(2):323-34. doi: 10.1042/BJ20110247. Biochem J. 2012. PMID: 22132725 - Structure, function and brain localization of neurotransmitter transporters.
Jursky F, Tamura S, Tamura A, Mandiyan S, Nelson H, Nelson N. Jursky F, et al. J Exp Biol. 1994 Nov;196:283-95. doi: 10.1242/jeb.196.1.283. J Exp Biol. 1994. PMID: 7823028 Review. - Glycine transporters: essential regulators of synaptic transmission.
Betz H, Gomeza J, Armsen W, Scholze P, Eulenburg V. Betz H, et al. Biochem Soc Trans. 2006 Feb;34(Pt 1):55-8. doi: 10.1042/BST0340055. Biochem Soc Trans. 2006. PMID: 16417482 Review.
Cited by
- Symmetrical dimer of the human dopamine transporter revealed by cross-linking Cys-306 at the extracellular end of the sixth transmembrane segment.
Hastrup H, Karlin A, Javitch JA. Hastrup H, et al. Proc Natl Acad Sci U S A. 2001 Aug 28;98(18):10055-60. doi: 10.1073/pnas.181344298. Proc Natl Acad Sci U S A. 2001. PMID: 11526230 Free PMC article. - Glycine transporter dimers: evidence for occurrence in the plasma membrane.
Bartholomäus I, Milan-Lobo L, Nicke A, Dutertre S, Hastrup H, Jha A, Gether U, Sitte HH, Betz H, Eulenburg V. Bartholomäus I, et al. J Biol Chem. 2008 Apr 18;283(16):10978-91. doi: 10.1074/jbc.M800622200. Epub 2008 Feb 5. J Biol Chem. 2008. PMID: 18252709 Free PMC article. - Structures and membrane interactions of native serotonin transporter in complexes with psychostimulants.
Yang D, Zhao Z, Tajkhorshid E, Gouaux E. Yang D, et al. Proc Natl Acad Sci U S A. 2023 Jul 18;120(29):e2304602120. doi: 10.1073/pnas.2304602120. Epub 2023 Jul 12. Proc Natl Acad Sci U S A. 2023. PMID: 37436958 Free PMC article. - Investigation of the biophysical and cell biological properties of ferroportin, a multipass integral membrane protein iron exporter.
Rice AE, Mendez MJ, Hokanson CA, Rees DC, Björkman PJ. Rice AE, et al. J Mol Biol. 2009 Feb 27;386(3):717-32. doi: 10.1016/j.jmb.2008.12.063. Epub 2009 Jan 3. J Mol Biol. 2009. PMID: 19150361 Free PMC article. - SLC6 transporter oligomerization.
Jayaraman K, Das AK, Luethi D, Szöllősi D, Schütz GJ, Reith MEA, Sitte HH, Stockner T. Jayaraman K, et al. J Neurochem. 2021 May;157(4):919-929. doi: 10.1111/jnc.15145. Epub 2020 Aug 28. J Neurochem. 2021. PMID: 32767560 Free PMC article. Review.
References
- Amara S G. Annu Rev Neurosci. 1993;16:73–93. - PubMed
- Nelson N. J Neurochem. 1998;71:1785–1803. - PubMed
- Schloss P, Püschel A, Betz H. Curr Opin Cell Biol. 1994;6:595–599. - PubMed
- Olivares L, Aragon C, Gimenez C, Zafra F. J Biol Chem. 1995;270:9437–9442. - PubMed
- Bennett E R, Kanner B I. J Biol Chem. 1997;272:1203–1210. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources