Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria - PubMed (original) (raw)
Platelet-mediated clumping of Plasmodium falciparum-infected erythrocytes is a common adhesive phenotype and is associated with severe malaria
A Pain et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Sequestration of malaria-infected erythrocytes in the peripheral circulation has been associated with the virulence of Plasmodium falciparum. Defining the adhesive phenotypes of infected erythrocytes may therefore help us to understand how severe disease is caused and how to prevent or treat it. We have previously shown that malaria-infected erythrocytes may form apparent autoagglutinates of infected erythrocytes. Here we show that such autoagglutination of a laboratory line of P. falciparum is mediated by platelets and that the formation of clumps of infected erythrocytes and platelets requires expression of the platelet surface glycoprotein CD36. Platelet-dependent clumping is a distinct adhesive phenotype, expressed by some but not all CD36-binding parasite lines, and is common in field isolates of P. falciparum. Finally, we have established that platelet-mediated clumping is strongly associated with severe malaria. Precise definition of the molecular basis of this intriguing adhesive phenotype may help to elucidate the complex pathophysiology of malaria.
Figures
Figure 1
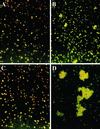
Clumping of infected erythrocytes in laboratory P. falciparum parasite clones. Clumps formed by ITO/C24 clone in PPP (A) and in PRP (B). Clumps formed by ITO/C10 clone in PPP (C) and in PRP (D).
Figure 2
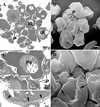
Transmission and scanning electron micrographs (TEMs and SEMs) of platelet-mediated clumping. (A) TEM section of a clump formed by parasite clone ITO/C10 in PRP shows several infected erythrocytes (denoted by letters IR) aggregated with interposed platelets. An SEM (B) at the same magnification shows several platelets (P) attached to the external surface of infected erythrocytes. (Bar represents 2 μm.) (C) A closer look at one of the clumps shows platelets (P) adherent to infected erythrocytes and acting as bridging cells between two parasitized erythrocytes (Pa). (Bar represents 1 μm.) At higher magnification (C, Inset) close apposition between platelets and ITO/C10 infected erythrocytes is seen at the electron-dense knob structures (indicated by the arrowheads). (Bar represents 0.5 μm.) (D) An SEM of an autoagglutinate in PRP showing close association between platelets and infected erythrocytes (denoted by arrows). (Bar represents 1 μm.)
Figure 3
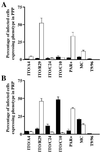
Expression of clumping and rosetting phenotypes of P. falciparum clones and lines (ITO/A4, ITO/R29, ITO/C24, ITO/C10, Palo Alto, Malayan Camp, and T9/96) in PPP (A) and in PRP (B) containing approximately 1 × 107 platelets per ml. Black bars and white bars represent clumping and rosetting frequencies, respectively, of the corresponding clones.
Figure 4
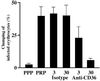
Inhibition of the clumping phenotype expressed by parasite clone ITO/C10 by using anti-CD36 mAb. Isotype indicates a control antibody. The numbers represent the concentration of the respective antibody used (3 μg ml−1 and 30 μg ml−1). The error bars represent standard error of mean from three independent experiments.
Figure 5
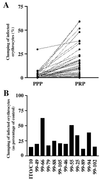
Platelet-mediated clumping among P. falciparum field isolates. (A) Clumping in parasite isolates from children with malaria in PPP and in PRP. These data sets represent 50 consecutive parasite isolates studied in 1999. (B) Inhibition of clumping in field parasite isolates by anti-CD36 mAb. The black bars represent the clumping frequencies of individual isolates when platelets were preincubated with anti-CD36 mAb compared with when platelets were preincubated with an isotype control antibody. The degree of inhibition of clumping in the laboratory clone ITO/C10 and in 11 consecutive field isolates that grew satisfactorily from frozen samples is shown.
References
- Roberts D D, Sherwood J A, Spitalnik S L, Panton L J, Howard R J, Dixit V M, Frazier W A, Miller L H, Ginsburg V. Nature (London) 1985;318:64–66. - PubMed
- Oquendo P, Hundt E, Lawler J, Seed B. Cell. 1989;58:95–101. - PubMed
- Ockenhouse C F, Tandon N N, Magowan C, Jamieson G A, Chulay J D. Science. 1989;243:1469–1471. - PubMed
- Berendt A R, Simmons D L, Tansey J, Newbold C I, Marsh K. Nature (London) 1989;341:57–59. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical