Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergic function - PubMed (original) (raw)
Genetic and pharmacological disruption of neurokinin 1 receptor function decreases anxiety-related behaviors and increases serotonergic function
L Santarelli et al. Proc Natl Acad Sci U S A. 2001.
Abstract
Alterations in serotonin (5-hydroxytriptamine, 5-HT), norepinephrine, and gamma-aminobutyric acid have been linked to the pathophysiology of anxiety and depression, and medications that modulate these neurotransmitters are widely used to treat mood disorders. Recently, the neuropeptide substance P (SP) and its receptor, the neurokinin 1 receptor (NK1R), have been proposed as possible targets for new antidepressant and anxiolytic therapies. However, animal and human studies have so far failed to provide a clear consensus on the role of SP in the modulation of emotional states. Here we show that both genetic disruption and acute pharmacological blockade of the NK1R in mice result in a marked reduction of anxiety and stress-related responses. These behavioral changes are paralleled by an increase in the firing rate of 5-HT neurons in the dorsal raphe nucleus, a major source of serotonergic input to the forebrain. NK1R disruption also results in a selective desensitization of 5-HT1A inhibitory autoreceptors, which resembles the effect of sustained antidepressant treatment. Together these results indicate that the SP system powerfully modulates anxiety and suggest that this effect is at least in part mediated by changes in the 5-HT system.
Figures
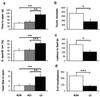
Figure 1
Decreased anxiety-related behavior in NK1 −/− mice. (a) EPM: time, percentage of entries, and number of head dips in the open arms (mean ± SEM). −/− animals show a significant increase in all three measures compared with +/+ and +/− mice (n = 12–14 mice per genotype). (b) Serum corticosterone was measured 30 min after a 10-min trial in the EPM. Bar graph represents percentage of increase in corticosterone levels from baseline levels measured 1 day before the EPM trial (mean ± SEM, n = 7 mice per genotype). (c) NSF paradigm: latency to feed (mean ± SEM, n = 16 mice per genotype). (d) USV: ultrasonic calls emitted by 8-day-old pups during a 2-min separation from the litter (mean ± SEM,n = 13 mice per genotype). Significant differences between +/+ (white bars), +/− (gray bars), and −/− (black bars) are marked by *, P < 0.05; **, P < 0.01; or ***, P < 0.001 (unpaired_t_ test).
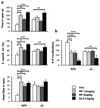
Figure 2
Effect of the NK1R antagonist RP67580 on anxiety-related behaviors. (a) EPM: diazepam (DZ) and both doses of RP67580 (RP) significantly decreased anxiety-related measures in +/+ mice, whereas only diazepam was effective in −/− (n = 9–10 mice group). (b) USV: the two doses of RP67580 produced a significant decrease in the number of vocalizations in +/+ mice whereas they had no effect in the −/− mice. Diazepam caused a significant decrease in vocalization in both genotypes. ANOVA revealed a main effect of treatment and an interaction of genotype and treatment in all measures presented. Significant differences between groups were calculated by Fisher post hoc analysis (*, P < 0.05; **, P < 0.01; or ***, P < 0.001).
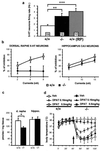
Figure 3
Effects of NK1R antagonism on 5-HT function. (a) The spontaneous firing rate (mean ± SEM, Hz) of 5-HT neurons in the DR was increased in the −/− mice and +/+ mice pretreated with RP67580 at a dose of 1.5 mg/kg. The numbers inside the bars indicate the number of cells tested. **, P < 0.01; ***, P < 0.001 (t test). (b) Percentage of inhibition of spontaneous firing of 5-HT neurons in the DR (Left) or quisqualate-induced firing of CA3 neurons in the hippocampus (Right) by microiontophoretic application of 8-OH-DPAT (mean ± SEM). The x axis indicates the intensity (in nAmp) of microiontophoretic currents used to deliver 8-OH-DPAT. 8-OH-DPAT has little effect on the firing of 5-HT neurons in −/− mice but produces a dose-dependent inhibition of firing in +/+ mice (Left). The ANOVA indicates a significant main effect of genotype (P < 0.01). The firing of CA3 neurons is equally inhibited by 8-OH-DPAT in the two genotypes (Right). (c) 8-OH-[3H]DPAT binding (mean ± SEM, pmol/mg of tissue) in DR and hippocampus. *, P < 0.05 (unpaired t test, n = 4–5). (d) Body temperature (mean ± SEM, n = 7). Arrow indicates the time of administration of either vehicle (Veh) or 8-OH-DPAT (DPAT) at the doses indicated. The repeated-measure ANOVA revealed a significant interaction of genotype and treatment (P < 0.01).
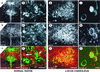
Figure 4
Localization of NK1Rs in the DR and LC. (a_–_f) Immunofluorescent double labeling of tryptophan hydroxylase (PH8, in green) and NK1R (in red) in the DR. The arrowhead indicates a neuron stained with the PH8 antibody (f), while the arrow shows a NK1R-positive neuron in the same area (f). [Scale bar: 200 μm (a_–_c) and 50 μm (d_–_f).] (g_–_l) Immunofluorescent double labeling of tyrosine hydroxylase (TH, in green) and NK1R (in red) in the LC. [Scale bar: 100 μm (g_–_i) and 10 μm (j_–_l).]
Similar articles
- Behavioral and physiologic effects of genetic or pharmacologic inactivation of the substance P receptor (NK1).
Santarelli L, Gobbi G, Blier P, Hen R. Santarelli L, et al. J Clin Psychiatry. 2002;63 Suppl 11:11-7. J Clin Psychiatry. 2002. PMID: 12562138 Review. - Interaction of GABA and serotonin in the anxiolytic action of diazepam and serotonergic anxiolytics.
López-Rubalcava C, Saldívar A, Fernández-Guasti A. López-Rubalcava C, et al. Pharmacol Biochem Behav. 1992 Oct;43(2):433-40. doi: 10.1016/0091-3057(92)90173-d. Pharmacol Biochem Behav. 1992. PMID: 1359576 - Substance P receptor antagonists in psychiatry: rationale for development and therapeutic potential.
Herpfer I, Lieb K. Herpfer I, et al. CNS Drugs. 2005;19(4):275-93. doi: 10.2165/00023210-200519040-00001. CNS Drugs. 2005. PMID: 15813642 Review. - Anxiolytic actions of the substance P (NK1) receptor antagonist L-760735 and the 5-HT1A agonist 8-OH-DPAT in the social interaction test in gerbils.
Cheeta S, Tucci S, Sandhu J, Williams AR, Rupniak NM, File SE. Cheeta S, et al. Brain Res. 2001 Oct 12;915(2):170-5. doi: 10.1016/s0006-8993(01)02846-3. Brain Res. 2001. PMID: 11595206
Cited by
- Neuronal nitric oxide synthase alteration accounts for the role of 5-HT1A receptor in modulating anxiety-related behaviors.
Zhang J, Huang XY, Ye ML, Luo CX, Wu HY, Hu Y, Zhou QG, Wu DL, Zhu LJ, Zhu DY. Zhang J, et al. J Neurosci. 2010 Feb 17;30(7):2433-41. doi: 10.1523/JNEUROSCI.5880-09.2010. J Neurosci. 2010. PMID: 20164327 Free PMC article. - Impact of substance P receptor antagonism on the serotonin and norepinephrine systems: relevance to the antidepressant/anxiolytic response.
Blier P, Gobbi G, Haddjeri N, Santarelli L, Mathew G, Hen R. Blier P, et al. J Psychiatry Neurosci. 2004 May;29(3):208-18. J Psychiatry Neurosci. 2004. PMID: 15173897 Free PMC article. - Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse.
Gadd CA, Murtra P, De Felipe C, Hunt SP. Gadd CA, et al. J Neurosci. 2003 Sep 10;23(23):8271-80. doi: 10.1523/JNEUROSCI.23-23-08271.2003. J Neurosci. 2003. PMID: 12967989 Free PMC article. - Evidence for mediation of nociception by injection of the NK-3 receptor agonist, senktide, into the dorsal periaqueductal gray of rats.
Bassi GS, Broiz AC, Gomes MZ, Brandão ML. Bassi GS, et al. Psychopharmacology (Berl). 2009 May;204(1):13-24. doi: 10.1007/s00213-008-1434-y. Epub 2008 Dec 18. Psychopharmacology (Berl). 2009. PMID: 19093101 - Attenuated Levels of Hippocampal Connexin 43 and its Phosphorylation Correlate with Antidepressant- and Anxiolytic-Like Activities in Mice.
Quesseveur G, Portal B, Basile JA, Ezan P, Mathou A, Halley H, Leloup C, Fioramonti X, Déglon N, Giaume C, Rampon C, Guiard BP. Quesseveur G, et al. Front Cell Neurosci. 2015 Dec 22;9:490. doi: 10.3389/fncel.2015.00490. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26733815 Free PMC article.
References
- De Felipe C, Herrero J F, O'Brien J A, Palmer J A, Doyle C A, Smith A J, Laird J M, Belmonte C, Cervero F, Hunt S P. Nature (London) 1998;392:394–397. - PubMed
- King T E, Heath M J, Debs P, Davis M B, Hen R, Barr G A. NeuroReport. 2000;11:587–591. - PubMed
- Cao Y Q, Mantyh P W, Carlson E J, Gillespie A M, Epstein C J, Basbaum A I. Nature (London) 1998;392:390–394. - PubMed
- Saria A. Eur J Pharmacol. 1999;375:51–60. - PubMed
- Holzer P. Neuroscience. 1988;24:739–768. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous