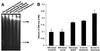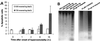Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells - PubMed (original) (raw)
Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells
D Kültz et al. Proc Natl Acad Sci U S A. 2001.
Abstract
This study demonstrates, by using neutral comet assay and pulsed field gel electrophoresis, that hyperosmotic stress causes DNA damage in the form of double strand breaks (dsb). Different solutes increase the rate of DNA dsb to different degrees at identical strengths of hyperosmolality. Hyperosmolality in the form of elevated NaCl (HNa) is most potent in this regard, whereas hyperosmolality in the form of elevated urea (HU) does not cause DNA dsb. The amount of DNA dsb increases significantly as early as 15 min after the onset of HNa. By using neutral comet and DNA ladder assays, we show that this rapid induction of DNA damage is not attributable to apoptosis. We demonstrate that renal inner medullary cells are able to efficiently repair hyperosmotic DNA damage within 48 h after exposure to hyperosmolality. DNA repair correlates with cell survival and is repressed by 25 microM LY294002, an inhibitor of DNA-activated protein kinases. These results strongly suggest that the hyperosmotic stress resistance of renal inner medullary cells is based not only on adaptations that protect cellular proteins from osmotic damage but, in addition, on adaptations that compensate DNA damage and maintain genomic integrity.
Figures
Figure 1
Induction of DNA dsb in mIMCD3 cells by HNa. Neutral comet assay was used to measure DNA dsb. (A) Control cells kept in isosmotic medium have only a short comet tail. (B) The comet tail increases after exposure of cells for 1 h to 725 mosmol/kg HNa. (C) Exposure of cells for 1 h to 5 milliunit/ml bleomycin also increases the comet tail. (D) Appearance of apoptotic cells when analyzed by neutral comet assay. Note the highly condensed nucleus and the very high ratio of comet tail length and width to nuclear diameter. (E) Quantification of comet moment (fraction of DNA in the comet tail versus the comet head) in mIMCD3 cells. Data are means ± SEM (n = 3).
Figure 2
HNa but not HU increases DNA dsb in mIMCD3 cells. PFGE was used to measure DNA dsb. (A) Representative PFGE gel illustrating the rate of migration of whole genomic DNA from cells exposed to isosmotic medium (300 mosmol/kg), 600 mosmol/kg HNa, 600 mosmol/kg HU, and 5 or 50 milliunit/ml bleomycin. (B) Quantification of DNA dsb by PFGE. The fraction of DNA recovered (FAR), which is the amount of DNA that has migrated from the well into the lane vs. total DNA in lane plus well, is plotted. Data shown are means ± SEM (n = 3).
Figure 3
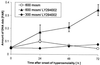
Kinetics and solute-specificity of hyperosmotic induction of DNA dsb in mIMCD3 cells. (A) Cells were exposed to hyperosmolality of 800 mosmol/kg for 15 min and 1 h. Hyperosmolality was because of addition of various solutes to regular growth medium. All solutes except urea increase DNA dsb rapidly (within 15 min). (B) DNA dsb after exposure of mIMCD3 cells to 600 mosmol/kg HNa are elevated for at least 18 h. Inhibition of DNA-activated protein kinases by LY294002 during exposure of cells to 600 mosmol/kg HNa leads to a significantly larger increase in DNA dsb (P < 0.05), indicating that these kinases are important for preventing DNA dsb from occurring or for their repair. Data are means ± SEM (n = 3).
Figure 4
Apoptosis cannot account for the increased DNA dsb during the early phase of hyperosmotic stress because its kinetics is slower than the induction of DNA dsb. (A) The percentage of apoptotic cells determined by neutral comet assay is low and not significantly different from isosmotic controls at all times when cells are exposed to 525 mosmol/kg HNa. Only after 15 h of exposure of cells to 725 mosmol/kg HNa does the number of apoptotic cells increase markedly. Data are means ± SEM (n = 3). (B) DNA ladder assay of mIMCD3 cells exposed to various degrees of HNa for 3 h. Equal amounts of whole genomic DNA were loaded in each well. Exposure of cells to 50 μg/ml etopside in serum-free medium served as a positive control for which the nucleosomal DNA ladder that is characteristic for apoptosis can be seen (last lane). DNA ladders are not present in genomic DNA of mIMCD3 cells exposed to HNa for 3 h, indicating that apoptosis is manifested at later times. Treatment with 25 μM LY294002 for 24 h did not induce apoptosis.
Figure 5
Solute-specific effects of inhibition of DNA-activated protein kinases on mIMCD3 cells exposed to hyperosmotic stress. (A) Morphology of mIMCD3 cells after growing them for 15 h in various media with or without 25 μM LY294002. (Magnification, ×400.) NaCl/urea 600 = 600 mosmol/kg HNa/HU, NaCl/urea 800 = 800 mosmol/kg HNa/HU. (B and C) Effect of LY294002 on number of cells left in a confluent 10-cm dish after 15 h culture in various osmolalities. (B) HNa. Data are means ± SEM (n = 3). Note that cell number drops significantly after exposure to 600 mosmol/kg with LY294002 but not without it. (C) HU. Data are means ± SEM (n = 3). Unlike with HNa, cell numbers are not significantly different between 300 and 600 mosmol/kg even in the presence of LY294002 during HU. When error bars are not visible, they are smaller than the corresponding symbol.
Figure 6
Suppression of cell growth and DNA repair by the DNA-activated protein kinase inhibitor LY294002. DNA dsb in mIMCD3 cells exposed to 600 mosmol/kg HNa are significantly increased at 24 h in isosmotic controls and in cells treated with 25 μM LY294002 plus HNa but not in cells treated with 25 μM LY294002 in isosmotic medium. DNA dsb continue to increase significantly when cells are left in HNa plus 25 μM LY294002, whereas they decrease to baseline by day 3 in HNa without LY294002. There is a small but significant increase in DNA dsb at 72 h when cells are exposed to 25 μM LY294002 in isosmotic medium. However, this increase is much smaller compared with the effect of LY294002 in HNa. DNA dsb were measured by PFGE; FAR = amount of DNA in lane/amount of DNA in (lane plus well). Data are means ± SEM (n = 3).
Similar articles
- Three GADD45 isoforms contribute to hypertonic stress phenotype of murine renal inner medullary cells.
Chakravarty D, Cai Q, Ferraris JD, Michea L, Burg MB, Kültz D. Chakravarty D, et al. Am J Physiol Renal Physiol. 2002 Nov;283(5):F1020-9. doi: 10.1152/ajprenal.00118.2002. Am J Physiol Renal Physiol. 2002. PMID: 12372778 - DNA damage and osmotic regulation in the kidney.
Dmitrieva NI, Burg MB, Ferraris JD. Dmitrieva NI, et al. Am J Physiol Renal Physiol. 2005 Jul;289(1):F2-7. doi: 10.1152/ajprenal.00041.2005. Am J Physiol Renal Physiol. 2005. PMID: 15951478 Review. - Hypertonic stress response.
Dmitrieva NI, Burg MB. Dmitrieva NI, et al. Mutat Res. 2005 Jan 6;569(1-2):65-74. doi: 10.1016/j.mrfmmm.2004.06.053. Mutat Res. 2005. PMID: 15603752 Review.
Cited by
- Serum urea concentration and risk of 16 site-specific cancers, overall cancer, and cancer mortality in individuals with metabolic syndrome: a cohort study.
Wu E, Wei GF, Li Y, Du MK, Ni JT. Wu E, et al. BMC Med. 2024 Nov 15;22(1):536. doi: 10.1186/s12916-024-03758-5. BMC Med. 2024. PMID: 39548477 Free PMC article. - Osmolar Modulation Drives Reversible Cell Cycle Exit and Human Pluripotent Cell Differentiation via NF-κВ and WNT Signaling.
Chui JS, Izuel-Idoype T, Qualizza A, de Almeida RP, Piessens L, van der Veer BK, Vanmarcke G, Malesa A, Athanasouli P, Boon R, Vriens J, van Grunsven L, Koh KP, Verfaillie CM, Lluis F. Chui JS, et al. Adv Sci (Weinh). 2024 Feb;11(7):e2307554. doi: 10.1002/advs.202307554. Epub 2023 Dec 1. Adv Sci (Weinh). 2024. PMID: 38037844 Free PMC article. - Direct ionic stress sensing and mitigation by the transcription factor NFAT5.
Khandwala CB, Sarkar P, Schmidt HB, Ma M, Kinnebrew M, Pusapati GV, Patel BB, Tillo D, Lebensohn AM, Rohatgi R. Khandwala CB, et al. bioRxiv [Preprint]. 2023 Sep 24:2023.09.23.559074. doi: 10.1101/2023.09.23.559074. bioRxiv. 2023. PMID: 37886503 Free PMC article. Preprint. - Solutions: how adaptive changes in cellular fluids enable marine life to cope with abiotic stressors.
Somero GN. Somero GN. Mar Life Sci Technol. 2022 Aug 16;4(3):389-413. doi: 10.1007/s42995-022-00140-3. eCollection 2022 Aug. Mar Life Sci Technol. 2022. PMID: 37073170 Free PMC article. Review. - A comparative high-resolution physicochemical analysis of commercially available fibrin sealants: Impact of sealant osmolality on biological performance.
Reichsöllner R, Heher P, Hartmann J, Manhartseder S, Singh R, Gulle H, Slezak P. Reichsöllner R, et al. J Biomed Mater Res A. 2023 Apr;111(4):488-501. doi: 10.1002/jbm.a.37466. Epub 2022 Nov 10. J Biomed Mater Res A. 2023. PMID: 36355631 Free PMC article.
References
- Somero G N, Yancey P. In: Osmolytes and Cell Volume Regulation: Physiological and Evolutionary Principles. Hoffmann J F, Jamieson J D, editors. Washington, DC: Am. Physiol. Soc.; 1997. pp. 441–484.
- Gullans S R, Cohen D M, Kojima R, Randall J, Brenner B M, Santos B, Chevaile A. Kidney Int. 1996;49:1678–1681. - PubMed
- Burg M B, Kwon E D, Kültz D. Annu Rev Physiol. 1997;59:437–455. - PubMed
- Kültz D, Madhany S, Burg M B. J Biol Chem. 1998;273:13645–13651. - PubMed
- Dmitrieva N, Kültz D, Michea L, Ferraris J D, Burg M B. J Biol Chem. 2000;275:18243–18247. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical