Cell vacuolation caused by Vibrio cholerae hemolysin - PubMed (original) (raw)
Cell vacuolation caused by Vibrio cholerae hemolysin
P Figueroa-Arredondo et al. Infect Immun. 2001 Mar.
Abstract
Non-O1 strains of Vibrio cholerae implicated in gastroenteritis and diarrhea generally lack virulence determinants such as cholera toxin that are characteristic of epidemic strains; the factors that contribute to their virulence are not understood. Here we report that at least one-third of diarrhea-associated nonepidemic V. cholerae strains from Mexico cause vacuolation of cultured Vero cells. Detailed analyses indicated that this vacuolation was related to that caused by aerolysin, a pore-forming toxin of Aeromonas; it involved primarily the endoplasmic reticulum at early times (approximately 1 to 4 h after exposure), and resulted in formation of large, acidic, endosome-like multivesicular vacuoles (probably autophagosomes) only at late times (approximately 16 h). In contrast to vacuolation caused by Helicobacter pylori VacA protein, that induced by V. cholerae was exacerbated by agents that block vacuolar proton pumping but not by endosome-targeted weak bases. It caused centripetal redistribution of endosomes, reflecting cytoplasmic alkalinization. The gene for V. cholerae vacuolating activity was cloned and was found to correspond to hlyA, the structural gene for hemolysin. HlyA protein is a pore-forming toxin that causes ion leakage and, ultimately, eukaryotic cell lysis. Thus, a distinct form of cell vacuolation precedes cytolysis at low doses of hemolysin. We propose that this vacuolation, in itself, contributes to the virulence of V. cholerae strains, perhaps by perturbing intracellular membrane trafficking or ion exchange in target cells and thereby affecting local intestinal inflammatory or other defense responses.
Figures
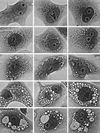
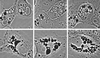
FIG. 1
Temporal progression of cell vacuolation caused by V. cholerae hemolysin (phase-contrast light microscopy; magnification, ×200). Top row, control cells, not exposed to toxin, showing no vacuolation. Second row, control for the exacerbation of _V. cholerae_-induced vacuolation by concanamycin (see row 4), showing that only a hint of vacuolation is caused by 1 h of exposure to this proton pump inhibitor alone. Third row, initial, relatively mild vacuolating effect caused by exposure to V. cholerae hemolysin for 1 to 2 h. Characteristically, this involves peripheral elements of the cell that can be identified as components of the ER by separate dye uptake and electron microscopic studies (not shown). Fourth row, more severe, diffuse, bubbly vacuolation caused by simultaneous 2-h exposure to V. cholerae hemolysin (same 1/500 dilution of culture supernatant as in row 3) plus 100 nM concanamycin (same concentration as in row 2). Fifth row, End-stage vacuolation of Vero cells, which appears in some cultures as early as 1 h after exposure to concentrated V. cholerae hemolysin but eventually is produced by all vacuolating strains at longer times of exposure to lower toxin concentrations. The gigantic vacuoles observed in such cells contain vast numbers of very negatively charged internal membranous vesicles, indicating they are multivesicular bodies or autophagosomes.
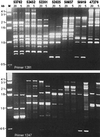
FIG. 2
RAPD fingerprinting of representative V. cholerae strains using two different informative primers, 1281 and 1247. The five-digit number above each pair of lanes indicates the strain tested. Both 20 and 5 ng of DNA were used in duplicate PCR to determine which apparent differences between strains truly reflect DNA sequence divergence rather than inhibitors in template DNA. An effect of an inhibitor is indicated in the 20-ng lane of the RAPD profile of strain 50919 with primer 1247. Lane M, 1-kb ladder.
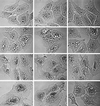
FIG. 3
Osmotic reversal of the vacuolating effect of V. cholerae hemolysin. Left column, after 3 h of exposure to a 1/500 dilution of V. cholerae supernatant, all cells are clearly vacuolated (the top two more diffusely than the lower one). Center column, addition of 300 mM sucrose to the medium of these same cells caused an immediate shrinkage of swollen ER in the upper two sets of cells (ones that happened to be exposed to the hemolysin alone) but no shrinkage of the larger, perinuclear vacuoles seen in the lower cell (one that was exposed additionally to concanamycin and hence progressed to the later, more severe stage of vacuolation [see Fig. 1, row 4]). Right column, the same cells, 2 min after washout of the 300 mM sucrose, showing prompt restoration of the diffuse vacuolation originally present in the top two swollen-ER type of cells (no further change is seen in the bottom cell).
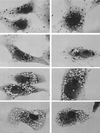
FIG. 4
The endocytotic tracer protein HRP is denied access to V. cholerae hemolysin-induced vacuoles, and thus they either are not derived from endosomes or are at least “off the circuit” of normal endosomal trafficking. Top row, control cells not exposed to V. cholerae hemolysin. After a 15-min exposure to HRP and a 30-min chase, the tracer has found its way deep into the cells, into the plethora of ∼0.5-μm granules that tend to collect in the perinuclear area, characteristic of normal late endosomes. Second row, cells loaded with HRP exactly like the control cells in the top row, but only after a 60-min exposure to a 1/500 dilution of V. cholerae supernatant. HRP endocytosis has proceeded to a normal extent (as judged by the numbers of dark granules that have formed), but the granules have remained dispersed throughout the cell, suggesting that they have failed to mature into late endosomes. (Parallel time-lapse light microscopic studies show that these endosomes lack the normal microtubule-directed motility that leads to their centripetal migration and maturation.) Note particularly that HRP has not gained access to any of the _V. cholerae_-induced vacuoles. Third row, cells prevacuolated by exposure to V. cholerae supernatant as in the second row and then exposed to HRP for only 5 min. Various amounts of HRP have entered these cells, from left to right panels, indicating different levels of endocytotic activity. At this early time of HRP entry, endosomes would not have had time to mature and move centripetally, even in normal cells. Hence, they remain scattered among the hemolysin-induced vacuoles but they still appear “unwilling” to fuse with these vacuoles. Bottom row, Controls for the above-described experiments, illustrating (in the left two panels) cells vacuolated with V. cholerae supernatant as described above and then treated for HRP histochemistry without ever exposing them to HRP, to ensure that the unusually large amount of DAB reaction product seen in some cells (such as the right panel of the third row) does not mean that Vero cells are somehow being stimulated by the hemolysin to express endogenous peroxidatic activity. The rightmost panel of this row shows cells exposed to toxin, then exposed to HRP, and then washed for a full 2 h to allow any recovery from vacuolation that might occur. Still, even at this late time point, HRP has not entered the remaining vacuoles.
FIG. 5
The dynamics of HRP-bearing endosomes indicate that V. cholerae hemolysin causes extreme cell alkalinization. Vero cells were prepared as described for Fig. 4, but were photographed with bright-field rather than phase-contrast microscopy to better see the dark DAB reaction product within HRP-loaded endosomes. Cell alkalinization is demonstrated by the fact that HRP-containing endosomes and lysosomes are drawn tightly to the very center of the cell, immediately around the MTOC which lies tight against the nucleus in such cultures. Top row, After 15 min of exposure to HRP, cells were exposed to a concentrated V. cholerae supernatant (1/250 dilution) for just 1 h. Note that such high doses of hemolysin produced only a very mild swelling of endosomes, and this was before the characteristic ER vacuolation began to occur. Second row, cells exposed for 3 h to a 1/500 dilution of V. cholerae supernatant, then exposed to HRP, and then exposed to the hemolysin again for an additional 30 min. This caused distinct cell alkalinization, with most endosomes drawn tightly into star-shaped aggregates immediately around the MTOCs. Third row, Cells again exposed to V. cholerae supernatant at a 1/500 dilution, but in the presence of concanamycin to exacerbate the vacuolation, before a 15-min exposure to HRP. This caused a slight inhibition of the centripetal movement of endosomes, probably due to physical blockage by the unusually enlarged vacuoles, but still permitted the formation of distinct accumulations of HRP-bearing endosomes at the MTOCs. Fourth row, Cells exposed to toxin in hypertonic sucrose, then exposed to HRP for 15 min, and then finally washed into normal Ringer's solution for 30 min after the HRP uptake. The presence of hypertonic sucrose retarded vacuole formation and allowed endosomes to move completely centrally to the MTOC, again indicating strong cell alkalinization. Subsequent release of the hypertonic protection by washing the cells into isotonic saline then allowed them to vacuolate severely. With this experimental protocol, the vacuoles end up devoid of HRP and distributed entirely peripheral to the tight central clusters of HRP-containing endosomes, further confirming that they are not endosomal in origin. (Note that in all four rows, bright-field viewing dramatizes the results shown in Fig. 4, namely, that V. cholerae's hemolysin-induced vacuoles do not take up any exogenous HRP.)
FIG. 6
Neutral red uptake in Vero cells vacuolated with V. cholerae hemolysin (top row) versus H. pylori VacA (bottom row). Top row, Vero cells were exposed to a 1/500 dilution of a V. cholerae supernatant for 3 h and then allowed to take up neutral red for 5 min. This stained only a subset of the vacuoles (presumably those that had advanced to be autophagic in nature). Bottom row, Vero cells were exposed to purified V. cholerae VacA toxin (1 μg/ml) for 16 h and then allowed to take up neutral red for 5 min. All vacuoles stained, indicating that all were strongly acidic and hence of endocytic origin.
FIG. 7
_Hin_dIII restriction fragment length polymorphism analysis of representative cosmid clones with vacuolating activity from the V. cholerae strain 52201 clone library. (A) Agarose gel electrophoresis of _Hin_dIII-digested DNAs. (B) Hybridization of _Hin_dIII digests with a 3-kb subclone with vacuolating activity (later shown to contain the hlyA gene). The largest hybridized band corresponded to the pLAFR-5 cosmid vector, and the other two bands (6.0 and 1.8 kb) contained sequences hybridizing to the 3-kb clone. No cosmid with vacuolating activity lacked these bands.
Similar articles
- Cell vacuolation, a manifestation of the El tor hemolysin of Vibrio cholerae.
Mitra R, Figueroa P, Mukhopadhyay AK, Shimada T, Takeda Y, Berg DE, Nair GB. Mitra R, et al. Infect Immun. 2000 Apr;68(4):1928-33. doi: 10.1128/IAI.68.4.1928-1933.2000. Infect Immun. 2000. PMID: 10722584 Free PMC article. - HlyA hemolysin of Vibrio cholerae O1 biotype E1 Tor. Identification of the hemolytic complex and evidence for the formation of anion-selective ion-permeable channels.
Menzl K, Maier E, Chakraborty T, Benz R. Menzl K, et al. Eur J Biochem. 1996 Sep 15;240(3):646-54. doi: 10.1111/j.1432-1033.1996.0646h.x. Eur J Biochem. 1996. PMID: 8856066 - Cytotoxic cell vacuolating activity from Vibrio cholerae hemolysin.
Coelho A, Andrade JR, Vicente AC, Dirita VJ. Coelho A, et al. Infect Immun. 2000 Mar;68(3):1700-5. doi: 10.1128/IAI.68.3.1700-1705.2000. Infect Immun. 2000. PMID: 10678992 Free PMC article. - [The comparative characteristics of Vibrio cholerae hemolysins].
Telesmanich NR. Telesmanich NR. Mikrobiol Zh (1978). 1992 Sep-Oct;54(5):105-12. Mikrobiol Zh (1978). 1992. PMID: 1453988 Review. Russian. - Epidemiology & molecular biology of Vibrio cholerae O139 Bengal.
Albert MJ. Albert MJ. Indian J Med Res. 1996 Jul;104:14-27. Indian J Med Res. 1996. PMID: 8783504 Review.
Cited by
- Cytoplasmic vacuolization in cell death and survival.
Shubin AV, Demidyuk IV, Komissarov AA, Rafieva LM, Kostrov SV. Shubin AV, et al. Oncotarget. 2016 Aug 23;7(34):55863-55889. doi: 10.18632/oncotarget.10150. Oncotarget. 2016. PMID: 27331412 Free PMC article. Review. - A polyvalent multiepitope protein cross-protects against Vibrio cholerae infection in rabbit colonization and passive protection models.
Upadhyay I, Li S, Ptacek G, Seo H, Sack DA, Zhang W. Upadhyay I, et al. Proc Natl Acad Sci U S A. 2022 Dec 13;119(50):e2202938119. doi: 10.1073/pnas.2202938119. Epub 2022 Dec 5. Proc Natl Acad Sci U S A. 2022. PMID: 36469767 Free PMC article. - The Vibrio cholerae cytolysin promotes activation of mast cell (T helper 2) cytokine production.
Arcidiacono D, Odom S, Frossi B, Rivera J, Paccani SR, Baldari CT, Pucillo C, Montecucco C, de Bernard M. Arcidiacono D, et al. Cell Microbiol. 2008 Apr;10(4):899-907. doi: 10.1111/j.1462-5822.2007.01092.x. Epub 2007 Nov 15. Cell Microbiol. 2008. PMID: 18005391 Free PMC article. - Hemolysin and the multifunctional autoprocessing RTX toxin are virulence factors during intestinal infection of mice with Vibrio cholerae El Tor O1 strains.
Olivier V, Haines GK 3rd, Tan Y, Satchell KJ. Olivier V, et al. Infect Immun. 2007 Oct;75(10):5035-42. doi: 10.1128/IAI.00506-07. Epub 2007 Aug 13. Infect Immun. 2007. PMID: 17698573 Free PMC article. - The extracellular metalloprotease of Vibrio tubiashii is a major virulence factor for pacific oyster (Crassostrea gigas) larvae.
Hasegawa H, Lind EJ, Boin MA, Häse CC. Hasegawa H, et al. Appl Environ Microbiol. 2008 Jul;74(13):4101-10. doi: 10.1128/AEM.00061-08. Epub 2008 May 2. Appl Environ Microbiol. 2008. PMID: 18456850 Free PMC article.
References
- Alm R A, Stroher U H, Manning P A. Extracellular proteins of Vibrio cholerae: nucleotide sequence of the structural gene (hlyA) for the haemolysin of the haemolytic El Tor strain O17 and characterization of the hlyA mutation in the non haemolytic classical strain 569B. Mol Microb. 1988;2:481–488. - PubMed
- Alm R A, Mayrhofer G, Kotlarski I, Manning P A. Amino-terminal domain of the El Tor haemolysin of Vibrio cholerae O1 is expressed in classical strains and is cytotoxic. Vaccine. 1991;9:588–594. - PubMed
- Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Short protocols in molecular biology. New York, N.Y: Wiley Interscience; 1989.
Publication types
MeSH terms
Substances
Grants and funding
- DK53727/DK/NIDDK NIH HHS/United States
- GM29647/GM/NIGMS NIH HHS/United States
- R01 GM029647/GM/NIGMS NIH HHS/United States
- AI38166/AI/NIAID NIH HHS/United States
- P30 DK52574/DK/NIDDK NIH HHS/United States
- P30 DK052574/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources