Human natural killer cells mediate killing of intracellular Mycobacterium tuberculosis H37Rv via granule-independent mechanisms - PubMed (original) (raw)
Human natural killer cells mediate killing of intracellular Mycobacterium tuberculosis H37Rv via granule-independent mechanisms
K J Brill et al. Infect Immun. 2001 Mar.
Abstract
Despite the continued importance of tuberculosis as a world-wide threat to public health, little is known about the mechanisms used by human lymphocytes to contain and kill the intracellular pathogen Mycobacterium tuberculosis. We previously described an in vitro model of infection of human monocytes (MN) with virulent M. tuberculosis strain H37Rv in which the ability of peripheral blood lymphocytes to limit intracellular growth of the organism could be measured. In the current study, we determined that lymphocyte-mediated killing of intracellular M. tuberculosis occurs within the first 24 h of coculture with infected MN. Natural killer (NK) cells isolated from both purified protein derivative (PPD)-positive and PPD-negative subjects were capable of mediating this early killing of intracellular H37Rv. NK cell-mediated killing of intracellular M. tuberculosis was not associated with the production of gamma interferon. Transferred supernatants of cocultured NK cells and M. tuberculosis-infected MN could not mediate the killing of intracellular M. tuberculosis, and Transwell studies indicated that direct cell-to-cell contact was required for NK cells to mediate the killing of the organism. Killing was not dependent upon exocytosis of NK cell cytotoxic granules. NK cells induced apoptosis of mycobacterium-infected MN, but neither killing of intracellular M. tuberculosis by NK cells nor NK cell-induced apoptosis of infected MN was inhibited by blocking the interaction of FasL and Fas. Thus, human NK cells may mediate killing of intracellular M. tuberculosis via alternative apoptotic pathways.
Figures
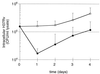
FIG. 1
PBL-mediated reduction in intracellular M. tuberculosis occurs within the first 24 h of coculture with infected MN. The CFU count of intracellular M. tuberculosis H37Rv within infected MN was determined at 24 h intervals for cultures of MN alone (○) and infected MN cocultured with PBL in a 10:1 PBL MN ratio (●). As illustrated, PBL mediated a drop in intracellular M. tuberculosis of approximately 1 log within the first 24 h of coculture, whereas in days 2 to 4 the rates of intracellular growth of the organism in the two culture conditions were comparable. The results represent the mean and the standard deviation (SD) from five donors.
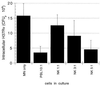
FIG. 2
NK cells mediate the killing of intracellular M. tuberculosis within 24 h of coculture with infected MN. CFU of intracellular M. tuberculosis was assessed in MN alone and following the addition of PBL (in a 10:1 ratio relative to MN) and of isolated CD3− CD56+ NK cells (in ratios of 1:1, 3:1, and 5:1 relative to infected MN). The results indicate the mean and SD for four subjects studied. As illustrated, NK cells were capable of killing intracellular M. tuberculosis. The magnitude of this effect increased with the use of higher ratios of NK to infected MN and was statistically significant following the addition of NK cells in both a 3:1 ratio (P = 0.006) and a 5:1 ratio (P = 0.003).
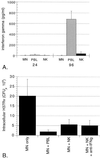
FIG. 3
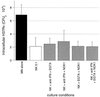
NK cells from both PPD-positive (A) and PPD-negative (B) subjects mediate a significant reduction in intracellular M. tuberculosis at 24 h. The addition of NK cells from PPD-positive donors to _M. tuberculosis_-infected MN resulted in a 63% decrease in intracellular H37Rv at 24 h, whereas the addition of NK cells from PPD-negative donors mediated a 70% reduction in the organism. The NK cell-mediated reduction in intracellular M. tuberculosis compared to CFU within MN alone was significant for both groups of subjects (P = 0.036 for PPD-positive subjects; P = 0.013 for PPD-negative subjects). The graphs illustrate the mean and SD for five subjects in each group.
FIG. 4
Killing of intracellular M. tuberculosis by NK cells is not mediated by IFN-γ. (A) Results of the measurement of IFN-γ within 24- and 96-h culture supernatants of _M. tuberculosis_-infected MN alone and infected MN cocultured with PBL or NK cells. As illustrated, none of the 24-h supernatants contained appreciable amounts of IFN-γ. At 96 h, PBL had produced significant amounts of IFN-γ in response to _M. tuberculosis_-infected MN. IFN-γ production by NK cells remained minimal, however, and was not significantly greater than within supernatants of infected MN alone. The data represent the mean and SD values for five subjects studied. (B) Effects of addition of blocking anti-IFN-γ polyclonal antibody (10 μg/ml) to cultures. As shown, blocking antibody had no effect on the NK cell-mediated killing of intracellular M. tuberculosis NK cells in medium alone induced a 72% reduction in intracellular M. tuberculosis, whereas in the presence of anti-IFN-γ, NK cells mediated a 75% reduction in intracellular H37Rv (P = 0.252 compared to NK cells in medium alone). The results represent the mean and SD of studies of cells obtained from five subjects.
FIG. 5
Supernatants of cocultured NK cells and _M. tuberculosis_-infected MN alone cannot mediate the killing of intracellular M. tuberculosis. As illustrated, the 92% reduction in intracellular M. tuberculosis mediated by the addition of NK cells (compared to the CFU level within infected MN alone) was much greater than that mediated by the addition of supernatants of cocultured NK cells and infected MN. Supernatants collected after 6, 18, and 24 h of coculture resulted in reductions in intracellular M. tuberculosis of only 13, 4, and 1.2%, respectively. The results represent the mean and SD studies from four subjects.
FIG. 6
NK cell-mediated killing of intracellular M. tuberculosis requires direct contact between NK cells and infected MN. The killing assay was modified to fit a 24-well dual-chamber Transwell plate format. The addition of NK cells directly to _M. tuberculosis_-infected MN within the lower chamber resulted in an 81% reduction in intracellular M. tuberculosis compared to the CFU count within MN alone. In contrast, the addition of NK cells to the top chamber of the Transwell plates, in which these effector cells were separated from infected MN by a 4-μm membrane, resulted in a nonsignificant 9.5% reduction in intracellular M. tuberculosis. The results represent the mean and SD of studies of four subjects.
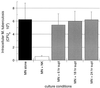
FIG. 7
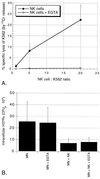
NK cell-mediated killing of intracellular M. tuberculosis is independent of the release of cytotoxic granules. (A) Purified NK cells in medium alone mediate the lysis of the standard K562 target leukemia cell line in a dose-dependent fashion (●), whereas in the presence of 5 mM EGTA this granule-dependent lysis is completely eliminated (○). The data represent the mean and SD of the percent specific lysis for four subjects studied. (B) Results of parallel studies of the effects of inhibition of granule release on the killing of intracellular M. tuberculosis. The reduction in intracellular M. tuberculosis by NK cells both in medium alone and in the presence of EGTA was statistically significant (P = 0.047 and P = 0.043, respectively). The figure illustrates the mean and SD for four subjects studied.
FIG. 8
Representative TUNEL assay showing ability of NK cells to induce apoptosis of BGC-infected human MN. MN apoptosis is indicated as percentage of CD 14-PE positive cells which are also TUNEL-FITC-positive. As illustrated in this example, BCG-infected MN alone display minimal apoptosis (A), whereas 18.9% of CD14+ cells become apoptotic during 24 hours of co-culture with NK cells (B). (Both dot plots represent data gated for monocytes from FSC/SSC images.)
FIG. 9
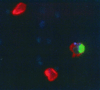
MN infected with virulent M. tuberculosis H37Rv undergo apoptosis in the presence of NK cells. H37Rv was stained with the fluorescent blue dye AlexaFluor 350 prior to infection. After 24 h of coculture with NK cells, MN were labeled with anti-CD14-PE, and cytospin slides were prepared for the in situ determination of apoptosis by using an FITC-labeled TUNEL reagent. As shown in this composite image, the addition of the smaller, unstained NK cells resulted in apoptosis (green) of the red-stained MN infected with (blue) AlexaFluor 350-stained H37Rv.
FIG. 10
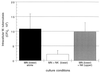
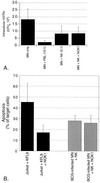
Both NK cell-mediated apoptosis of BCG-infected MN and killing of intracellular M. tuberculosis are independent of Fas-FasL interactions. Anti-FasL blocking antibody NOK1 (10 μg/ml) was added to cultures containing infected MN and NK cells. Panel A shows the mean and the SD for CFU studies of five subjects. As illustrated, the addition of NOK1 had no effect on the ability of NK cells to kill intracellular H37Rv. Likewise, although NOK1 did inhibit the FasL-dependent induction of apoptosis of Jurkat cells by the FasL-transfectant KFL9 cell line, NK cell-mediated apoptosis of MN-infected M. bovis BCG was not affected by the addition of NOK1, as shown in panel B. Jurkat-KFL9 results represent the mean and SD from triplicate experiments. MN-NK data is presented as mean and SD from studies of four subjects.
FIG. 11
NK cell-mediated killing of intracellular M. tuberculosis cannot be accounted for by interactions of IFN-γ, cytotoxic granule release, and Fas-FasL interactions. To assess the possibility that the additive effects of these pathways were required in the killing of M. tuberculosis by NK cells, neutralizing anti-IFN-γ monoclonal antibody, EGTA, and NOK1 (anti-FasL) were added to cocultures of NK cells and infected MN in combination. As shown, none of the combinations of two or all three of the inhibitors led to a significant decrease in the ability of NK cells to kill intracellular M. tuberculosis. The results represent the mean and SD for five subjects studied.
Similar articles
- CD4(+) T cell and natural killer cell-dependent killing of Mycobacterium tuberculosis by human monocytes.
Yoneda T, Ellner JJ. Yoneda T, et al. Am J Respir Crit Care Med. 1998 Aug;158(2):395-403. doi: 10.1164/ajrccm.158.2.9707102. Am J Respir Crit Care Med. 1998. PMID: 9700112 - Bovine natural killer cells restrict the replication of Mycobacterium bovis in bovine macrophages and enhance IL-12 release by infected macrophages.
Denis M, Keen DL, Parlane NA, Storset AK, Buddle BM. Denis M, et al. Tuberculosis (Edinb). 2007 Jan;87(1):53-62. doi: 10.1016/j.tube.2006.03.005. Epub 2006 May 26. Tuberculosis (Edinb). 2007. PMID: 16730232 - The mechanism of organophosphorus pesticide-induced inhibition of cytolytic activity of killer cells.
Li Q, Kawada T. Li Q, et al. Cell Mol Immunol. 2006 Jun;3(3):171-8. Cell Mol Immunol. 2006. PMID: 16893497 Review. - Activation of NK cell cytotoxicity.
Smyth MJ, Cretney E, Kelly JM, Westwood JA, Street SE, Yagita H, Takeda K, van Dommelen SL, Degli-Esposti MA, Hayakawa Y. Smyth MJ, et al. Mol Immunol. 2005 Feb;42(4):501-10. doi: 10.1016/j.molimm.2004.07.034. Mol Immunol. 2005. PMID: 15607806 Review.
Cited by
- Changes in leucocyte and lymphocyte subsets during tuberculosis treatment; prominence of CD3dimCD56+ natural killer T cells in fast treatment responders.
Veenstra H, Baumann R, Carroll NM, Lukey PT, Kidd M, Beyers N, Bolliger CT, van Helden PD, Walzl G. Veenstra H, et al. Clin Exp Immunol. 2006 Aug;145(2):252-60. doi: 10.1111/j.1365-2249.2006.03144.x. Clin Exp Immunol. 2006. PMID: 16879244 Free PMC article. - Natural killer cells as a therapeutic tool for infectious diseases - current status and future perspectives.
Schmidt S, Tramsen L, Rais B, Ullrich E, Lehrnbecher T. Schmidt S, et al. Oncotarget. 2018 Apr 17;9(29):20891-20907. doi: 10.18632/oncotarget.25058. eCollection 2018 Apr 17. Oncotarget. 2018. PMID: 29755697 Free PMC article. Review. - Natural killer cells: a coherent model for their functional role in Mycobacterium tuberculosis infection.
Esin S, Batoni G. Esin S, et al. J Innate Immun. 2015;7(1):11-24. doi: 10.1159/000363321. Epub 2014 Sep 2. J Innate Immun. 2015. PMID: 25196698 Free PMC article. Review. - Antibacterial role for natural killer cells in host defense to Bacillus anthracis.
Gonzales CM, Williams CB, Calderon VE, Huante MB, Moen ST, Popov VL, Baze WB, Peterson JW, Endsley JJ. Gonzales CM, et al. Infect Immun. 2012 Jan;80(1):234-42. doi: 10.1128/IAI.05439-11. Epub 2011 Oct 17. Infect Immun. 2012. PMID: 22006566 Free PMC article. - Nonpathogenic SIV and Pathogenic HIV Infections Associate with Disparate Innate Cytokine Signatures in Response to Mycobacterium bovis BCG.
Gasper MA, Biswas SP, Fisher BS, Ehnert SC, Sherman DR, Sodora DL. Gasper MA, et al. PLoS One. 2016 Aug 9;11(8):e0158149. doi: 10.1371/journal.pone.0158149. eCollection 2016. PLoS One. 2016. PMID: 27505158 Free PMC article.
References
- Balcewicz-Sablinska M K, Keane J, Kornfeld H, Remold H G. Pathogenic Mycobacterium tuberculosis evades apoptosis of host macrophages by release of TNF-R2, resulting in inactivation of TNFα. J Immunol. 1998;161:2636–2641. - PubMed
- Bancroft G. The role of natural killer cells in innate resistance to infection. Curr Opin Immunol. 1993;5:503–510. - PubMed
- Bancroft G J, Bosma M J, Bosma G C, Unanue E R. Regulation of macrophage Ia expression in mice with severe combined immunodeficiency: induction of Ia expression by a T cell independent mechanism. J Immunol. 1986;137:4–9. - PubMed
- Barnes P F, Grisso C L, Abranms J S, Band H, Rea T H, Modlin R L. γδ T lymphoyctes in human tuberculosis. J Infect Dis. 1992;165:506–512. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI27243/AI/NIAID NIH HHS/United States
- AI36219/AI/NIAID NIH HHS/United States
- AI35027/AI/NIAID NIH HHS/United States
- K08 AI001581/AI/NIAID NIH HHS/United States
- AI01581/AI/NIAID NIH HHS/United States
- R01 AI027243/AI/NIAID NIH HHS/United States
- HL59851/HL/NHLBI NIH HHS/United States
- N01AI95383/AI/NIAID NIH HHS/United States
- P30 AI036219/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous