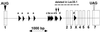Requirement of the Lec35 gene for all known classes of monosaccharide-P-dolichol-dependent glycosyltransferase reactions in mammals - PubMed (original) (raw)
Requirement of the Lec35 gene for all known classes of monosaccharide-P-dolichol-dependent glycosyltransferase reactions in mammals
M Anand et al. Mol Biol Cell. 2001 Feb.
Free PMC article
Abstract
The Lec35 gene product (Lec35p) is required for utilization of the mannose donor mannose-P-dolichol (MPD) in synthesis of both lipid-linked oligosaccharides (LLOs) and glycosylphosphatidylinositols, which are important for functions such as protein folding and membrane anchoring, respectively. The hamster Lec35 gene is shown to encode the previously identified cDNA SL15, which corrects the Lec35 mutant phenotype and predicts a novel endoplasmic reticulum membrane protein. The mutant hamster alleles Lec35.1 and Lec35.2 are characterized, and the human Lec35 gene (mannose-P-dolichol utilization defect 1) was mapped to 17p12-13. To determine whether Lec35p was required only for MPD-dependent mannosylation of LLO and glycosylphosphatidylinositol intermediates, two additional lipid-mediated reactions were investigated: MPD-dependent C-mannosylation of tryptophanyl residues, and glucose-P-dolichol (GPD)-dependent glucosylation of LLO. Both were found to require Lec35p. In addition, the SL15-encoded protein was selective for MPD compared with GPD, suggesting that an additional GPD-selective Lec35 gene product remains to be identified. The predicted amino acid sequence of Lec35p does not suggest an obvious function or mechanism. By testing the water-soluble MPD analog mannose-beta-1-P-citronellol in an in vitro system in which the MPD utilization defect was preserved by permeabilization with streptolysin-O, it was determined that Lec35p is not directly required for the enzymatic transfer of mannose from the donor to the acceptor substrate. These results show that Lec35p has an essential role for all known classes of monosaccharide-P-dolichol-dependent reactions in mammals. The in vitro data suggest that Lec35p controls an aspect of MPD orientation in the endoplasmic reticulum membrane that is crucial for its activity as a donor substrate.
Figures
Figure 1
Exon-intron organization of the hamster Lec35 gene. A fragment of normal CHO-K1 genomic DNA, ∼5 kbp, was amplified by LA-PCR (Takara Shuzo, Tokyo, Japan) with 5′ UTR and 3′ UTR primers from SL15. The fragment was gel-purified and used directly for automated DNA sequence analysis on both strands by using various sequencing primers suggested by SL15 cDNA sequence (GenBank accession U55387) as well as sequence obtained from introns. Exon-intron boundaries were deduced by directly comparing the genomic and cDNA sequences. Exon X was identified in a rare nonfunctional clone from the original cDNA library (Ware et al., 1996). Inclusion of exon X altered the 5′ splice junction of exon 4. Rodent genomic repetitive elements (Table 1; Jurka_et al._, 1996) are indicated by horizontal arrowheads: a, B1 class; b, B2 class; c, B3 class; d, RSINE1 class. Two_Bam_HI sites identified by sequencing and used for restriction enzyme digests are noted by the downward vertical arrows. Translation initiation (AUG) and termination (UAG) sites are shown by vertical arrowheads. The entire annotated sequence has been deposited with GenBank (accession AF250376). A region extending from within intron 1 through exon X that appears deleted in the Lec35.1 allele (see text) is indicated. Because the Lec35.1 mutation is recessive, it is highly likely that the parental cell that gave rise to the Lec35.1 mutant had a single copy of a normal allele at the Lec35 locus. It is highly unlikely that the exact same deletion occurred twice in a single cell starting with two normal Lec35 alleles.
Figure 2
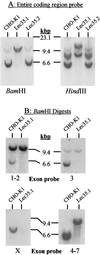
Analysis of Lec35 nucleic acids. Southern blot analysis of genomic DNA. 32P probes were generated with specific PCR primers and covered either the complete coding sequence of SL15 cDNA (A), or exons 1 and 2, exon 3, exon X, or exons 4–7 (B). Genomic DNA (Easy-DNA; Invitrogen, San Diego, CA) from parental CHO-K1, Lec35.1, or Lec35.2 cells (10 μg for all probes except exon X [50 μg]) was digested with either _Bam_HI or_Hin_dIII as indicated and analyzed by Southern blots according to standard methods (Sambrook et al., 1989). The positions of λ phage-_Hin_dIII markers are shown. In addition to a strongly hybridizing fragment of ∼6 kbp, a faint_Bam_HI fragment of ∼10 kbp was reproducibly detected with the exon 3 probe in CHO-K1 DNA. Because exon 3 lacks a_Bam_HI site, this is likely to be an irrelevant cross-reacting fragment. Northern blot analysis of RNA. Total RNA was isolated from the CHO-K1, Lec35.1, or Lec35.2 cells lines, as well as the 6C and 10A transfectants of Lec35.1 grown in the absence or presence of 10 ng/ml doxycyclin for 4 d, and 10 μg of total RNA was analyzed by gel electrophoresis and blotting (C and D) as described (Sambrook et al., 1989). A 32P probe prepared from the complete coding sequence of SL15 cDNA was used. Relative intensities of the Lec35 mRNA bands, as described in the text, were measured with a phosphorimager (Fuji, Stamford, CT). The migrations of native (n) and recombinant (r) Lec35 transcripts (D) were reproducible. The position of a 1.35-kb RNA marker (Life Technologies) is shown (C).
Figure 2
Analysis of Lec35 nucleic acids. Southern blot analysis of genomic DNA. 32P probes were generated with specific PCR primers and covered either the complete coding sequence of SL15 cDNA (A), or exons 1 and 2, exon 3, exon X, or exons 4–7 (B). Genomic DNA (Easy-DNA; Invitrogen, San Diego, CA) from parental CHO-K1, Lec35.1, or Lec35.2 cells (10 μg for all probes except exon X [50 μg]) was digested with either _Bam_HI or_Hin_dIII as indicated and analyzed by Southern blots according to standard methods (Sambrook et al., 1989). The positions of λ phage-_Hin_dIII markers are shown. In addition to a strongly hybridizing fragment of ∼6 kbp, a faint_Bam_HI fragment of ∼10 kbp was reproducibly detected with the exon 3 probe in CHO-K1 DNA. Because exon 3 lacks a_Bam_HI site, this is likely to be an irrelevant cross-reacting fragment. Northern blot analysis of RNA. Total RNA was isolated from the CHO-K1, Lec35.1, or Lec35.2 cells lines, as well as the 6C and 10A transfectants of Lec35.1 grown in the absence or presence of 10 ng/ml doxycyclin for 4 d, and 10 μg of total RNA was analyzed by gel electrophoresis and blotting (C and D) as described (Sambrook et al., 1989). A 32P probe prepared from the complete coding sequence of SL15 cDNA was used. Relative intensities of the Lec35 mRNA bands, as described in the text, were measured with a phosphorimager (Fuji, Stamford, CT). The migrations of native (n) and recombinant (r) Lec35 transcripts (D) were reproducible. The position of a 1.35-kb RNA marker (Life Technologies) is shown (C).
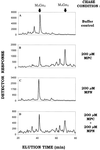
Figure 3
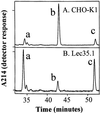
C-mannosylation of RNase 2.4. RNase 2.4 was expressed in either CHO-K1 (A) or Lec35.1 (B) cells, purified from the conditioned medium, digested with thermolysin at 75°C, and the peptides were fractionated by reversed phase C18 HPLC. Only the portion of the chromatogram where peptides from the N terminus of the protein eluted has been shown. Peak b contains the C-mannosylated peptide [FT(C2-Man-)WAQW], whereas peaks a and c contain two forms of the unmodified peptide, TWAQW and FTWAQW.
Figure 4
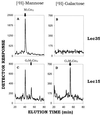
Absence of Glc3Man5GlcNAc2-P-P-dolichol in Lec35 mutant cells. Lec35.1 cells (A and B) and Lec15.2 cells (Camp_et al._, 1993) (C and D) were labeled with 1 mCi/ml [2-3H]mannose (A and C) or [1-3H]galactose (B and D) for 15 min as described under MATERIALS AND METHODS. Lipid-linked oligosaccharides were extracted, and the labeled oligosaccharides were released by mild acid hydrolysis and analyzed by HPLC.
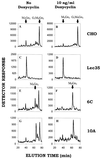
Figure 5
Glucosylation of Man9GlcNAc2-P-P-dolichol in vivo depends upon the level of expression of Lec35 mRNA. CHO-K1 (A and B), Lec 35.1 (C and D), 6C (E and F), or 10A cells (G and H) were grown in the absence (A, C, E, and G) or presence (B, D, F, and H) of 10 ng/ml doxycyclin for 4 d, and labeled with 1 mCi/ml [2-3H]mannose for 15 min. Oligosaccharides were obtained from the LLO fraction by mild acid hydrolysis and analyzed by HPLC.
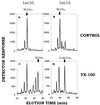
Figure 6
Mild perturbation efficiently corrects defective glycosylation in Lec35 cells. Lec35.1 (A and C) and Lec15.2 (B and D) cells were incubated with [3H]mannose to label endogenous Man5GlcNAc2-P-P-dolichol. Cells were treated in the absence (A and B; control) or presence (C and D; TX-100) of 0.05% (wt/vol) Triton X-100, and then incubated with 0.1 mM unlabeled GDP-mannose. The oligosaccharides were recovered and compared with known standards, as indicated, by HPLC. Note that Man5GlcNAc2-P-P-dolichol in treated Lec15 cells (D) is converted to Glc3Man5GlcNAc2-P-P-dolichol, but additional mannose residues are not added.
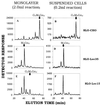
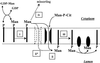
Figure 7
Streptolysin-O permeabilizes Lec35 cells and preserves the mannosylation defect. CHO-K1 (A and B), Lec35.1 (C and D), and Lec15.2 (E and F) cells were treated with SLO either when attached to dishes (Monolayer, A, C, and E) or after suspension (Suspended Cells, B, D, and F). The permeabilized cells were incubated with either 2.0 ml (A, C, and E) or 0.2 ml (B, D, and F) of transport buffer (Martys et al., 1995) at 37°C for 10 min containing 1 μM unlabeled UDP-GlcNAc to initiate LLO synthesis, 0.1 μCi GDP-[3H]mannose to label and extend LLOs, and 0.2 mM 5′ AMP to inhibit breakdown of nucleotide sugars. LLO were recovered and characterized by HPLC.
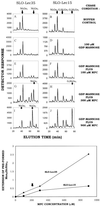
Figure 8
Mannose-P-citronellol is an efficient mannose donor in SLO-treated Lec35 cells. (Upper left) Lec35.1 (A, C, E, G, and I) and Lec15.2 (B, D, F, H, and J) cells were labeled with [3H]mannose. They were treated with SLO, and then incubated for 10 min with transport buffer (A and B), or buffer plus 100 μM unlabeled GDP-mannose (to convert any [3H]Man1-4GlcNAc2-P-P-dolichol to [3H]Man5GlcNAc2-P-P-dolichol) and either 0 μM (C and D), 100 μM (E and F), 300 μM (G and H), or 900 μM (I and J) unlabeled MPC. LLO were recovered and analyzed by HPLC. Note that Man9GlcNAc2-P-P-dolichol is glucosylated by endogenous GPD more effectively in SLO-Lec15 cells than SLO-Lec35 cells, as expected. (Lower left) HPLC data from the upper left figure were processed (see MATERIALS AND METHODS) to give a normalized, quantitative value for the amount of MPC-dependent mannosylation that occurred at each concentration. The maximum possible value is 4.0. The downward arrow indicates the MPC concentration used in the upper right figure. It is not known why MPC-treated SLO-Lec35 cells had higher values than SLO-Lec15 cells. (Upper right) Lec35.1 (A, C, E, G, and I) and Lec15.2 (B, D, F, H, and J) cells were labeled with [3H]mannose and treated with SLO in a manner comparable to that shown in the upper left figure. The cells were then incubated in buffer alone for 10 min (A and B), or buffer with unlabeled 100 μM GDP-mannose and 200 μM MPC for either 5 min (C and D), 10 min (E and F), 20 min (G and H), or 30 min (I and J). LLO were analyzed by HPLC. (Lower right) The data from the upper right figure were processed to gives values for the extent of MPC-dependent mannosylation, as done for the lower left figure. The downward arrow shows the time used for incubations in the upper left figure.
Figure 8
Mannose-P-citronellol is an efficient mannose donor in SLO-treated Lec35 cells. (Upper left) Lec35.1 (A, C, E, G, and I) and Lec15.2 (B, D, F, H, and J) cells were labeled with [3H]mannose. They were treated with SLO, and then incubated for 10 min with transport buffer (A and B), or buffer plus 100 μM unlabeled GDP-mannose (to convert any [3H]Man1-4GlcNAc2-P-P-dolichol to [3H]Man5GlcNAc2-P-P-dolichol) and either 0 μM (C and D), 100 μM (E and F), 300 μM (G and H), or 900 μM (I and J) unlabeled MPC. LLO were recovered and analyzed by HPLC. Note that Man9GlcNAc2-P-P-dolichol is glucosylated by endogenous GPD more effectively in SLO-Lec15 cells than SLO-Lec35 cells, as expected. (Lower left) HPLC data from the upper left figure were processed (see MATERIALS AND METHODS) to give a normalized, quantitative value for the amount of MPC-dependent mannosylation that occurred at each concentration. The maximum possible value is 4.0. The downward arrow indicates the MPC concentration used in the upper right figure. It is not known why MPC-treated SLO-Lec35 cells had higher values than SLO-Lec15 cells. (Upper right) Lec35.1 (A, C, E, G, and I) and Lec15.2 (B, D, F, H, and J) cells were labeled with [3H]mannose and treated with SLO in a manner comparable to that shown in the upper left figure. The cells were then incubated in buffer alone for 10 min (A and B), or buffer with unlabeled 100 μM GDP-mannose and 200 μM MPC for either 5 min (C and D), 10 min (E and F), 20 min (G and H), or 30 min (I and J). LLO were analyzed by HPLC. (Lower right) The data from the upper right figure were processed to gives values for the extent of MPC-dependent mannosylation, as done for the lower left figure. The downward arrow shows the time used for incubations in the upper left figure.
Figure 9
Mannose-P-nerol is not a mannose donor with SLO-treated Lec35 cells. Lec35.1 cells were incubated with [3H]mannose and treated with SLO as in Figure 8. The cells were then incubated in buffer containing 100 μM unlabeled GDP-mannose alone (A), with MPC (B), with MPN (C), or with MPC plus MPN (D), each at a concentration of 200 μM. LLO were then analyzed by HPLC.
Figure 10
Possible sites of action of Lec35 protein. A schematic representation of MPD-dependent mannosylation reactions in the ER membrane is shown. Lec35 protein (Lec35p) is required for mannosylation of at least three classes of acceptor substrate (LLO, GPI, tryptophan residues of specific proteins) represented as a black oval oriented at the lumenal side of the ER membrane. Reactions that do not require Lec35p directly are shown by solid arrows, whereas reactions that might involve Lec35p are indicated by dotted arrows. The experiments reported here show that the catalytic transfer of mannose from MPD to acceptor does not directly involve Lec35p, and previous work established that MPD synthesis did not require Lec35p. As explained in the INTRODUCTION, any proposed mechanism must have the potential to account for correction of the Lec35 phenotype by physical or chemical perturbation. (i) Lec35p might be involved on the cytoplasmic leaflet in movement or exchange of MPD from MPD synthase to the MPD flippase (indicated by paired trapezoids). (ii) Lec35p might be involved in MPD flippase function, perhaps as the flippase itself, a regulatory subunit, or a regulator of its expression. As discussed in the text, it appears unlikely that Lec35p is involved in the function of the flippase-like activity assayed by MPC transport. However, there still remains a possibility of other uncharacterized MPD flippase activities (ii*) that require Lec35p. (iii) Lec35p might be involved on the lumenal leaflet in movement or exchange of MPD from the flippase to the various mannosyltransferases. (iv) Lec35p might be necessary to prevent missorting of MPD to an enzymatically inactive domain of the ER membrane, or to another organelle.
Similar articles
- Expression cloning of a novel suppressor of the Lec15 and Lec35 glycosylation mutations of Chinese hamster ovary cells.
Ware FE, Lehrman MA. Ware FE, et al. J Biol Chem. 1996 Jun 14;271(24):13935-8. doi: 10.1074/jbc.271.24.13935. J Biol Chem. 1996. PMID: 8663248 - Defective mannosylation of glycosylphosphatidylinositol in Lec35 Chinese hamster ovary cells.
Camp LA, Chauhan P, Farrar JD, Lehrman MA. Camp LA, et al. J Biol Chem. 1993 Mar 25;268(9):6721-8. J Biol Chem. 1993. PMID: 8454644 - Unexpected basis for impaired Glc3Man9GlcNAc2-P-P-dolichol biosynthesis by elevated expression of GlcNAc-1-P transferase.
Gao N, Shang J, Lehrman MA. Gao N, et al. Glycobiology. 2008 Jan;18(1):125-34. doi: 10.1093/glycob/cwm109. Epub 2007 Oct 3. Glycobiology. 2008. PMID: 17913728 - Dolichol-phosphate mannose synthase: structure, function and regulation.
Maeda Y, Kinoshita T. Maeda Y, et al. Biochim Biophys Acta. 2008 Jun;1780(6):861-8. doi: 10.1016/j.bbagen.2008.03.005. Epub 2008 Mar 14. Biochim Biophys Acta. 2008. PMID: 18387370 Review. - Enzymes that recognize dolichols participate in three glycosylation pathways and are required for protein secretion.
Orlean P. Orlean P. Biochem Cell Biol. 1992 Jun;70(6):438-47. doi: 10.1139/o92-067. Biochem Cell Biol. 1992. PMID: 1333231 Review.
Cited by
- Cystinosin, the protein defective in cystinosis, is a H(+)-driven lysosomal cystine transporter.
Kalatzis V, Cherqui S, Antignac C, Gasnier B. Kalatzis V, et al. EMBO J. 2001 Nov 1;20(21):5940-9. doi: 10.1093/emboj/20.21.5940. EMBO J. 2001. PMID: 11689434 Free PMC article. - The clinical relevance of glycobiology.
Schachter H. Schachter H. J Clin Invest. 2001 Dec;108(11):1579-82. doi: 10.1172/JCI14498. J Clin Invest. 2001. PMID: 11733552 Free PMC article. No abstract available. - Complexity of the eukaryotic dolichol-linked oligosaccharide scramblase suggested by activity correlation profiling mass spectrometry.
Verchère A, Cowton A, Jenni A, Rauch M, Häner R, Graumann J, Bütikofer P, Menon AK. Verchère A, et al. Sci Rep. 2021 Jan 14;11(1):1411. doi: 10.1038/s41598-020-80956-0. Sci Rep. 2021. PMID: 33446867 Free PMC article. - Nonenzymatic synthesis of anomerically pure, mannosyl-based molecular probes for scramblase identification studies.
Picca G, Probst M, Langenegger SM, Khorev O, Bütikofer P, Menon AK, Häner R. Picca G, et al. Beilstein J Org Chem. 2020 Jul 20;16:1732-1739. doi: 10.3762/bjoc.16.145. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32765793 Free PMC article. - AglR is required for addition of the final mannose residue of the N-linked glycan decorating the Haloferax volcanii S-layer glycoprotein.
Kaminski L, Guan Z, Abu-Qarn M, Konrad Z, Eichler J. Kaminski L, et al. Biochim Biophys Acta. 2012 Oct;1820(10):1664-70. doi: 10.1016/j.bbagen.2012.06.014. Epub 2012 Jun 27. Biochim Biophys Acta. 2012. PMID: 22750201 Free PMC article.
References
- Burda P, Jakob CA, Beinhauer J, Hegemann JH, Aebi M. Ordered assembly of the asymmetrically branched lipid-linked oligosaccharide in the endoplasmic reticulum is ensured by the substrate specificity of the individual glycosyltransferases. Glycobiology. 1999;9:617–625. - PubMed
- Camp LA, Chauhan P, Farrar JD, Lehrman MA. Defective mannosylation of glycosylphosphatidylinositol in Lec35 Chinese hamster ovary cells. J Biol Chem. 1993;268:6721–6728. - PubMed
- Chapman A, Fujimoto K, Kornfeld S. The primary glycosylation defect in class E thy-1-negative mutant mouse lymphoma cells is an inability to synthesize dolichol-P-mannose. J Biol Chem. 1980;255:4441–4446. - PubMed
- Chapman A, Trowbridge IS, Hyman R, Kornfeld S. Structure of the lipid-linked oligosaccharides that accumulate in class E thy-1-negative mutant lymphomas. Cell. 1979;17:509–515. - PubMed
- Cipollo JF, Trimble RB. The accumulation of Man6GlcNAc2-PP-dolichol in the Saccharomyces cerevisiaealg9 mutant reveals a regulatory role for the Alg3p alpha1,3-Man middle-arm addition in downstream oligosaccharide-lipid and glycoprotein glycan processing. J Biol Chem. 2000;275:4267–4277. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- GM-36065/GM/NIGMS NIH HHS/United States
- R56 GM038545/GM/NIGMS NIH HHS/United States
- GM-38545/GM/NIGMS NIH HHS/United States
- R01 GM038545/GM/NIGMS NIH HHS/United States
- R01 GM036065/GM/NIGMS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases