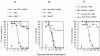Immunotherapy of a human papillomavirus type 16 E7-expressing tumor by administration of fusion protein comprised of Mycobacterium bovis BCG Hsp65 and HPV16 E7 - PubMed (original) (raw)
Immunotherapy of a human papillomavirus type 16 E7-expressing tumor by administration of fusion protein comprised of Mycobacterium bovis BCG Hsp65 and HPV16 E7
N R Chu et al. Cell Stress Chaperones. 2000 Nov.
Abstract
Human papillomavirus type 16 (HPV16) infection has been linked to the development of cervical and anal dysplasia and cancer. One hallmark of persistent infection is the synthesis of the viral E7 protein in cervical epithelial cells. The expression of E7 in dysplastic and transformed cells and its recognition by the immune system as a foreign antigen make it an ideal target for immunotherapy. Utilizing the E7-expressing murine tumor cell line, TC-1, as a model of cervical carcinoma, an immunotherapy based on the administration of an adjuvant-free fusion protein comprised of Mycobacterium bovis BCG Hsp65 linked to HPV16 E7 (HspE7) has been developed. Initial in vitro analyses indicate that immunization with HspE7 results in the induction of a type 1 immune response based on the pattern of secreted cytokines and the presence of cytolytic activity following antigenic recall. It has been previously shown that prophylactic immunization with HspE7 protected mice against challenge with TC-1 cells and that these tumor-free animals are also protected against rechallenge with TC-1 cells. The present report shows that a single therapeutic immunization with HspE7 induces regression of palpable tumors, confers protection against tumor rechallenge, and is associated with long-term survival (>253 days). In vivo studies using mice with targeted mutations in CD8 or MHC class II or depleted of CD8 or CD4 lymphocyte subsets demonstrate that tumor regression following therapeutic HspE7 immunization is CD8 dependent and CD4 independent. These studies extend previous observations on the induction of CTL by Hsp fusion proteins and are consistent with the clinical application of HspE7 as an immunotherapy for human cervical and anal dysplasia and cancer.
Figures
Fig 1.
A single therapeutic immunization with HspE7 induces TC-1 tumor regression and promotes long-term survival. C57BL/6 mice were injected SC in the hind flank with 1.3 × 105 TC-1 cells (day 0). Seven days postimplantation, when measurable tumor (2 × 2-mm minimum) was apparent in all animals, mice were arbitrarily assigned into groups (17 to 18 animals per group) and immunized SC in the scruff of the neck with either PBS, 1.4 nmol of (h)E7, or 1.4 nmol HspE7 (arrow). The same cohorts of PBS (O), (h)E7 (▵), or HspE7 (▪)-treated mice were used to produce the data displayed in (A), (B), and (C). (A) Percentage tumor incidence in mice receiving HspE7 therapy. Mice were scored for the presence or absence of a palpable SC tumor nodule for 50 days postimplantation. (B) Individual tumor volumes from mice receiving HspE7 therapy. Tumor volumes of SC nodules were assessed for 50 days postimplantation. (C) Long-term survival in mice receiving HspE7 therapy. Mice were monitored for survival over a 253-day period postimplantation. Mice that became moribund because of tumor burden were sacrificed. Time to death is plotted on a Kaplan-Meier survival curve (reprinted with permission; see author's note).
Fig 2.
Tumor regression following HspE7 immunization is CD8 dependent and CD4 independent. (A) and (B) Antibody-depleted or nondepleted mice were injected SC in the hind flank with 1.3 × 105 TC-1 cells (day 0). Seven days postimplantation (arrow), a depleted and nondepleted group of mice (12 to 15 animals per group) were treated with 1.4 nmol HspE7, as indicated in the legend. A third group was treated with PBS. The presence of SC tumor was monitored for 37 or 39 days. Data are presented as percent tumor incidence per group. (A) CD8+ T cell–depleted mice using MoAb 53.6-72; (B) CD4+ T cell-depleted mice using MoAb GK1.5. (C) Mice with a targeted mutation in CD8α (CD8 KO) or the β chain of Ab (class II KO) were implanted with 1.3 × 105 TC-1 cells SC in the hind flank. Seven days later (arrow), the CD8 KO and class II KO cohorts were divided into 2 groups (9 to 10 per group) and immunized with PBS or 1.4 nmol HspE7, as indicated in the legend. The presence of SC tumor was monitored for 42 days. Data are presented as percentage tumor incidence per group (reprinted with permission; see author's note)
Similar articles
- Immunotherapy of a human papillomavirus (HPV) type 16 E7-expressing tumour by administration of fusion protein comprising Mycobacterium bovis bacille Calmette-Guérin (BCG) hsp65 and HPV16 E7.
Chu NR, Wu HB, Wu T, Boux LJ, Siegel MI, Mizzen LA. Chu NR, et al. Clin Exp Immunol. 2000 Aug;121(2):216-25. doi: 10.1046/j.1365-2249.2000.01293.x. Clin Exp Immunol. 2000. PMID: 10931134 Free PMC article. - Induction of CD4-independent E7-specific CD8+ memory response by heat shock fusion protein.
Liu H, Wu BH, Rowse GJ, Emtage PC. Liu H, et al. Clin Vaccine Immunol. 2007 Aug;14(8):1013-23. doi: 10.1128/CVI.00029-07. Epub 2007 Jun 27. Clin Vaccine Immunol. 2007. PMID: 17596433 Free PMC article. - CD4+ T cell-mediated antigen-specific immunotherapy in a mouse model of cervical cancer.
Daniel D, Chiu C, Giraudo E, Inoue M, Mizzen LA, Chu NR, Hanahan D. Daniel D, et al. Cancer Res. 2005 Mar 1;65(5):2018-25. doi: 10.1158/0008-5472.CAN-04-3444. Cancer Res. 2005. PMID: 15753402 - Technology evaluation: HspE7 (Stressgen).
Maciag PC, Paterson Y. Maciag PC, et al. Curr Opin Mol Ther. 2005 Jun;7(3):256-63. Curr Opin Mol Ther. 2005. PMID: 15977424 Review. - The need for improvement of the treatment of advanced and metastatic cervical cancer, the rationale for combined chemo-immunotherapy.
van Meir H, Kenter GG, Burggraaf J, Kroep JR, Welters MJ, Melief CJ, van der Burg SH, van Poelgeest MI. van Meir H, et al. Anticancer Agents Med Chem. 2014 Feb;14(2):190-203. doi: 10.2174/18715206113136660372. Anticancer Agents Med Chem. 2014. PMID: 24237223 Review.
Cited by
- Heat shock proteins as emerging therapeutic targets.
Sõti C, Nagy E, Giricz Z, Vígh L, Csermely P, Ferdinandy P. Sõti C, et al. Br J Pharmacol. 2005 Nov;146(6):769-80. doi: 10.1038/sj.bjp.0706396. Br J Pharmacol. 2005. PMID: 16170327 Free PMC article. Review. - Heat shock fusion protein-based immunotherapy for treatment of cervical intraepithelial neoplasia III.
Einstein MH, Kadish AS, Burk RD, Kim MY, Wadler S, Streicher H, Goldberg GL, Runowicz CD. Einstein MH, et al. Gynecol Oncol. 2007 Sep;106(3):453-60. doi: 10.1016/j.ygyno.2007.04.038. Epub 2007 Jun 22. Gynecol Oncol. 2007. PMID: 17586030 Free PMC article. Clinical Trial. - Full eradication of pre-clinical human papilloma virus-induced tumors by a lentiviral vaccine.
Douguet L, Fert I, Lopez J, Vesin B, Le Chevalier F, Moncoq F, Authié P, Nguyen TM, Noirat A, Névo F, Blanc C, Bourgine M, Hardy D, Anna F, Majlessi L, Charneau P. Douguet L, et al. EMBO Mol Med. 2023 Oct 11;15(10):e17723. doi: 10.15252/emmm.202317723. Epub 2023 Sep 7. EMBO Mol Med. 2023. PMID: 37675835 Free PMC article. - CpG-conjugated apoptotic tumor cells elicit potent tumor-specific immunity.
Shirota H, Klinman DM. Shirota H, et al. Cancer Immunol Immunother. 2011 May;60(5):659-69. doi: 10.1007/s00262-011-0973-y. Epub 2011 Feb 4. Cancer Immunol Immunother. 2011. PMID: 21318638 Free PMC article. - Prophylactic Antitumor Effect of Mixed Heat Shock Proteins/Peptides in Mouse Sarcoma.
Wang Y, Liu SY, Yuan M, Tang Y, Guo QY, Cui XM, Sui X, Peng J. Wang Y, et al. Chin Med J (Engl). 2015 Aug 20;128(16):2234-41. doi: 10.4103/0366-6999.162516. Chin Med J (Engl). 2015. PMID: 26265619 Free PMC article.
References
- Anthony LSD, Wu H, Sweet H, Turnnir C, Boux LJ, Mizzen LA. Priming of CD8+ CTL effector cells in mice by immunization with a stress protein-influenza virus nucleoprotein fusion molecule. Vaccine. 1998;17:373–383. - PubMed
- Bennett SRM, Carbone FR, Karamalis F, Flavell RA, Miller JFAP, Heath WR. Help for cytotoxic-T-cell responses is mediated by CD40 signalling. Nature. 1998;393:478–480. - PubMed
- Bosch FX, Manos MM, Munoz N, et al. Prevalence of human papillomavirus in cervical cancer: a worldwide perspective. J Natl Cancer Inst. 1995;87:796–802. - PubMed
- Cho BK, Palliser D, Guillen E, Wisniewski J, Young RA, Chen J, Eisen HN. A proposed mechanism for the induction of cytotoxic T lymphocyte production by heat shock fusion proteins. Immunity. 2000;12:263–272. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials