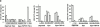Caspase activation is required for terminal erythroid differentiation - PubMed (original) (raw)
Caspase activation is required for terminal erythroid differentiation
Y Zermati et al. J Exp Med. 2001.
Abstract
The cysteine proteases known as caspases play a central role in most apoptotic pathways. Here, we show that caspase inhibitors arrest the maturation of human erythroid progenitors at early stages of differentiation, before nucleus and chromatin condensation. Effector caspases such as caspase-3 are transiently activated through the mitochondrial pathway during erythroblast differentiation and cleave proteins involved in nucleus integrity (lamin B) and chromatin condensation (acinus)without inducing cell death and cleavage of GATA-1. These observations indicate a new function for caspases as key proteases in the process of erythroid differentiation.
Figures
Figure 1
The caspase inhibitor z-VAD-fmk arrests erythroid maturation at the basophilic stage. CD36 cells (2 × 105 cells/ml) were cultured for 8 d in the presence of Epo or Epo + TGF-β1 with (+) or without (−) 150 μM z-VAD-fmk (ZVAD). Every 2 or 3 d, cells were diluted to 2 × 105 cells/ml, and cytokines and z-VAD-fmk were added. (A) Cell morphology was examined after May-Grünwald-Giemsa staining on day 8 of the culture. Absolute numbers of each type of erythroblasts (immature cells, mature cells, and erythrocytes) in the presence or absence of z-VAD-fmk are indicated above percentages. (B) Percentage of cells containing hemoglobin was estimated after benzidine staining. All results are the mean of four independent experiments.
Figure 1
The caspase inhibitor z-VAD-fmk arrests erythroid maturation at the basophilic stage. CD36 cells (2 × 105 cells/ml) were cultured for 8 d in the presence of Epo or Epo + TGF-β1 with (+) or without (−) 150 μM z-VAD-fmk (ZVAD). Every 2 or 3 d, cells were diluted to 2 × 105 cells/ml, and cytokines and z-VAD-fmk were added. (A) Cell morphology was examined after May-Grünwald-Giemsa staining on day 8 of the culture. Absolute numbers of each type of erythroblasts (immature cells, mature cells, and erythrocytes) in the presence or absence of z-VAD-fmk are indicated above percentages. (B) Percentage of cells containing hemoglobin was estimated after benzidine staining. All results are the mean of four independent experiments.
Figure 2
Caspase-3, -2, and -9 are transiently activated, and PARP is cleaved during erythroid differentiation. DEVD-AFC peptide cleavage activity (caspase-3/-7) was monitored in whole cell lysates from 0.5 × 106 CD36+ cells cultured for indicated times (day 2, 3, 5, 7; d2, 3, 5, 7) in the presence of Epo or Epo + TGF-β1. Addition of 150 μM z-VAD-fmk (ZVAD) in all conditions inhibited caspases activation. Results are expressed as mean percentage ± SD (n = 3) of the activity measured in cells deprived for 12 h of growth factors (Deprived). (B and C) Immunoblots were performed on whole cell lysates from 0.5 × 106 CD36+ cells either deprived of growth factors for 12 h (Deprived) or cultured for indicated times in the presence of Epo to detect the 32-kD procaspase-3 and its p20, p19, and p17 cleavage products (B) or the native 116-kD PARP (PARP-p116) and its 85-kD cleavage product (PARP-p85) (C). Arrowheads indicate localization of specific products. HSP90 is shown as a loading control. (D) CD36 cells (2 × 105 cells/ml) were cultured in the presence of Epo with (+) or without (−) 150 μM DEVD-cmk (DEVD). Every 2 or 3 d, cells were diluted to 2 × 105 cells/ml, and cytokines and DEVD-cmk were added. Cell morphology was examined after May-Grünwald-Giemsa staining on day 8 of the culture. (E) The hydrolysis of IETD-AFC (caspase-8), LEHD-AFC (caspase-9), and VDVAD-AFC (caspase-2) peptide substrates was monitored in whole cell lysates from 0.5 × 106 CD36+ cells cultured for indicated times in the presence of Epo or Epo + TGF-β1. For caspase-3, -9, and -2 activation; results are expressed as percentage of the activity measured in 12-h growth factor–deprived cells (Deprived), and results for caspase-8 activity (A) are expressed as percentage of the activity measured in lysates of cells cultured with Epo + 500 ng/mL of an agonist anti-CD95 (Fas) mAb (Fas-L) for 12 h.
Figure 2
Caspase-3, -2, and -9 are transiently activated, and PARP is cleaved during erythroid differentiation. DEVD-AFC peptide cleavage activity (caspase-3/-7) was monitored in whole cell lysates from 0.5 × 106 CD36+ cells cultured for indicated times (day 2, 3, 5, 7; d2, 3, 5, 7) in the presence of Epo or Epo + TGF-β1. Addition of 150 μM z-VAD-fmk (ZVAD) in all conditions inhibited caspases activation. Results are expressed as mean percentage ± SD (n = 3) of the activity measured in cells deprived for 12 h of growth factors (Deprived). (B and C) Immunoblots were performed on whole cell lysates from 0.5 × 106 CD36+ cells either deprived of growth factors for 12 h (Deprived) or cultured for indicated times in the presence of Epo to detect the 32-kD procaspase-3 and its p20, p19, and p17 cleavage products (B) or the native 116-kD PARP (PARP-p116) and its 85-kD cleavage product (PARP-p85) (C). Arrowheads indicate localization of specific products. HSP90 is shown as a loading control. (D) CD36 cells (2 × 105 cells/ml) were cultured in the presence of Epo with (+) or without (−) 150 μM DEVD-cmk (DEVD). Every 2 or 3 d, cells were diluted to 2 × 105 cells/ml, and cytokines and DEVD-cmk were added. Cell morphology was examined after May-Grünwald-Giemsa staining on day 8 of the culture. (E) The hydrolysis of IETD-AFC (caspase-8), LEHD-AFC (caspase-9), and VDVAD-AFC (caspase-2) peptide substrates was monitored in whole cell lysates from 0.5 × 106 CD36+ cells cultured for indicated times in the presence of Epo or Epo + TGF-β1. For caspase-3, -9, and -2 activation; results are expressed as percentage of the activity measured in 12-h growth factor–deprived cells (Deprived), and results for caspase-8 activity (A) are expressed as percentage of the activity measured in lysates of cells cultured with Epo + 500 ng/mL of an agonist anti-CD95 (Fas) mAb (Fas-L) for 12 h.
Figure 3
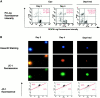
Mitochondrial transmembrane potential (ΔΨ) is disrupted during erythroid differentiation. For indicated times, ΔΨ disruption was assessed (A) by cytometer analysis after cell labeling with DiOC6(3) and PI. Cells with disruption of ΔΨ were DiOC6(3) negative and PI negative. A positive control of apoptosis was obtained by cytokines deprivation of CD36+ cells for 12 h (Deprived) (B) by fluorescence microscopy and cytometric analysis of cells labeled with the JC-1 fluorescent dye. Hoescht 33342 staining showed nucleus morphology.
Figure 4
Caspase activation does not induce either phosphatidylserine externalization or GATA-1 cleavage. (A) Cells cultured for 5 d in the presence of Epo or Epo + TGF-β1 with (+ZVAD) or without (−ZVAD) 150 μM z-VAD-fmk were labeled with annexin V and PI as described earlier. As negative and positive controls, cells were deprived of cytokines for 12 h (Deprived) with or without z-VAD-fmk, respectively. (B) Expression of the 46-kD erythroid transcription factor GATA-1 was studied by immunoblot in whole cell lysates from 0.5 × 106 CD36+ cells deprived of growth factor for 12 h (Deprived) or cultured for indicated times with Epo.
Figure 4
Caspase activation does not induce either phosphatidylserine externalization or GATA-1 cleavage. (A) Cells cultured for 5 d in the presence of Epo or Epo + TGF-β1 with (+ZVAD) or without (−ZVAD) 150 μM z-VAD-fmk were labeled with annexin V and PI as described earlier. As negative and positive controls, cells were deprived of cytokines for 12 h (Deprived) with or without z-VAD-fmk, respectively. (B) Expression of the 46-kD erythroid transcription factor GATA-1 was studied by immunoblot in whole cell lysates from 0.5 × 106 CD36+ cells deprived of growth factor for 12 h (Deprived) or cultured for indicated times with Epo.
Figure 5
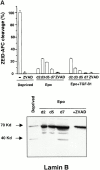
Cleavage of acinus and lamin B but not DFF45/ICAD during erythroid differentiation. (A) Top: hydrolysis of ZEID-AFC was monitored in whole cell lysates from 0.5 × 106 CD36+ cells cultured for indicated times in the presence of Epo or Epo + TGF-β1. Results are expressed as mean percentage ± SD (n = 3) of the activity measured in cells deprived for 12 h of growth factors (Deprived). Addition of z-VAD-fmk 150 μM (ZVAD) inhibited caspase-6 activation in all conditions of culture. Bottom: immunoblot analysis of the 70-kD lamin B and its 45-kD cleavage product in whole cell lysates from 0.5 × 106 CD36+ cells deprived of growth factor for 12 h (Deprived), cultured for indicated times with Epo, or cultured for 5 d with Epo + 150 μM z-VAD-fmk (+ZVAD). (B) Immunoblot analysis of the 98-kD protein acinus, its 23-kD cleavage product, and the 48-kD DFF45/ICAD protein in whole cell lysates from CD36+ cells cultured for indicated times with Epo or Epo + 150 μM z-VAD-fmk for 5 d (+ZVAD), or with Epo + TGF-β1 for 2 d. As positive control of apoptosis, cells were deprived with growth factors for 12 h (Deprived).
Figure 5
Cleavage of acinus and lamin B but not DFF45/ICAD during erythroid differentiation. (A) Top: hydrolysis of ZEID-AFC was monitored in whole cell lysates from 0.5 × 106 CD36+ cells cultured for indicated times in the presence of Epo or Epo + TGF-β1. Results are expressed as mean percentage ± SD (n = 3) of the activity measured in cells deprived for 12 h of growth factors (Deprived). Addition of z-VAD-fmk 150 μM (ZVAD) inhibited caspase-6 activation in all conditions of culture. Bottom: immunoblot analysis of the 70-kD lamin B and its 45-kD cleavage product in whole cell lysates from 0.5 × 106 CD36+ cells deprived of growth factor for 12 h (Deprived), cultured for indicated times with Epo, or cultured for 5 d with Epo + 150 μM z-VAD-fmk (+ZVAD). (B) Immunoblot analysis of the 98-kD protein acinus, its 23-kD cleavage product, and the 48-kD DFF45/ICAD protein in whole cell lysates from CD36+ cells cultured for indicated times with Epo or Epo + 150 μM z-VAD-fmk for 5 d (+ZVAD), or with Epo + TGF-β1 for 2 d. As positive control of apoptosis, cells were deprived with growth factors for 12 h (Deprived).
Similar articles
- [Erythropoiesis: a paradigm for the role of caspases in cell death and differentiation].
Ribeil JA, Zermati Y, Vandekerckhove J, Dussiot M, Kersual J, Hermine O. Ribeil JA, et al. J Soc Biol. 2005;199(3):219-31. doi: 10.1051/jbio:2005023. J Soc Biol. 2005. PMID: 16471262 Review. French. - Control of erythroid cell production via caspase-mediated cleavage of transcription factor SCL/Tal-1.
Zeuner A, Eramo A, Testa U, Felli N, Pelosi E, Mariani G, Srinivasula SM, Alnemri ES, Condorelli G, Peschle C, De Maria R. Zeuner A, et al. Cell Death Differ. 2003 Aug;10(8):905-13. doi: 10.1038/sj.cdd.4401255. Cell Death Differ. 2003. PMID: 12867998 - Negative regulation of erythropoiesis by caspase-mediated cleavage of GATA-1.
De Maria R, Zeuner A, Eramo A, Domenichelli C, Bonci D, Grignani F, Srinivasula SM, Alnemri ES, Testa U, Peschle C. De Maria R, et al. Nature. 1999 Sep 30;401(6752):489-93. doi: 10.1038/46809. Nature. 1999. PMID: 10519553 - Caspase activation in the terminal differentiation of human epidermal keratinocytes.
Weil M, Raff MC, Braga VM. Weil M, et al. Curr Biol. 1999 Apr 8;9(7):361-4. doi: 10.1016/s0960-9822(99)80162-6. Curr Biol. 1999. PMID: 10209121 - Apoptotic mechanisms in the control of erythropoiesis.
Testa U. Testa U. Leukemia. 2004 Jul;18(7):1176-99. doi: 10.1038/sj.leu.2403383. Leukemia. 2004. PMID: 15208642 Review.
Cited by
- Apoptosis Inhibitor 5: A Multifaceted Regulator of Cell Fate.
Abbas H, Derkaoui DK, Jeammet L, Adicéam E, Tiollier J, Sicard H, Braun T, Poyet JL. Abbas H, et al. Biomolecules. 2024 Jan 22;14(1):136. doi: 10.3390/biom14010136. Biomolecules. 2024. PMID: 38275765 Free PMC article. Review. - CK2β Regulates Hematopoietic Stem Cell Biology and Erythropoiesis.
Quotti Tubi L, Canovas Nunes S, Mandato E, Pizzi M, Vitulo N, D'Agnolo M, Colombatti R, Martella M, Boaro MP, Doriguzzi Breatta E, Fregnani A, Spinello Z, Nabergoj M, Filhol O, Boldyreff B, Albiero M, Fadini GP, Gurrieri C, Vianello F, Semenzato G, Manni S, Trentin L, Piazza F. Quotti Tubi L, et al. Hemasphere. 2023 Nov 23;7(12):e978. doi: 10.1097/HS9.0000000000000978. eCollection 2023 Dec. Hemasphere. 2023. PMID: 38026791 Free PMC article. - SARS-CoV-2 Impact on Red Blood Cell Morphology.
Kondratov KA, Artamonov AA, Mikhailovskii VY, Velmiskina AA, Mosenko SV, Grigoryev EA, Anisenkova AY, Nikitin YV, Apalko SV, Sushentseva NN, Ivanov AM, Scherbak SG. Kondratov KA, et al. Biomedicines. 2023 Oct 26;11(11):2902. doi: 10.3390/biomedicines11112902. Biomedicines. 2023. PMID: 38001903 Free PMC article. - How does caspases regulation play role in cell decisions? apoptosis and beyond.
Ghorbani N, Yaghubi R, Davoodi J, Pahlavan S. Ghorbani N, et al. Mol Cell Biochem. 2024 Jul;479(7):1599-1613. doi: 10.1007/s11010-023-04870-5. Epub 2023 Nov 17. Mol Cell Biochem. 2024. PMID: 37976000 Review. - Evidence of interactions among apoptosis, cell proliferation, and dedifferentiation in the rudiment during whole-organ intestinal regeneration in the sea cucumber.
Reyes-Rivera J, Grillo-Alvarado V, Soriano-López AE, García-Arrarás JE. Reyes-Rivera J, et al. Dev Biol. 2024 Jan;505:99-109. doi: 10.1016/j.ydbio.2023.11.001. Epub 2023 Nov 3. Dev Biol. 2024. PMID: 37925124
References
- Gregory C.J., Eaves A.C. Three stages of erythropoietic progenitor cell differentiation distinguished by a number of physical and biologic properties. Blood. 1978;51:527–537. - PubMed
- Krantz S.B. Erythropoietin. Blood. 1991;77:419–434. - PubMed
- Socolovsky M., Fallon A.E., Wang S., Brugnara C., Lodish H.F. Fetal anemia and apoptosis of red cell progenitors in Stat5a-/-5b-/- micea direct role for Stat5 in Bcl-X(L) induction. Cell. 1999;98:181–191. - PubMed
- Gregoli P.A., Bondurant M.C. Function of caspases in regulating apoptosis caused by erythropoietin deprivation in erythroid progenitors. J. Cell. Physiol. 1999;178:133–143. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous